Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Dermatological indications
Off-label uses
How it works
Precautions
Side effects
Use in specific populations
Bimatoprost is a prostaglandin analogue sold in the US, Canada and Europe by the pharmaceutical company Allergan. It was first marketed under the trade name Lumigan® (bimatoprost ophthalmic solution 0.01% or 0.03%) to reduce intraocular pressure in patients with glaucoma or ocular hypertension.
Researchers noticed the drug had the side effect of stimulating eyelash growth and darkening of the eyelashes in patients with glaucoma.
Allergan re-applied for US Food and Drug Administration (FDA) approval for bimatoprost for the cosmetic purpose of eyelash growth under the new name Latisse®. In December 2008, bimatoprost ophthalmic solution 0.03% was approved by the FDA as a treatment for eyelash hypotrichosis (inadequate or not enough eyelashes).
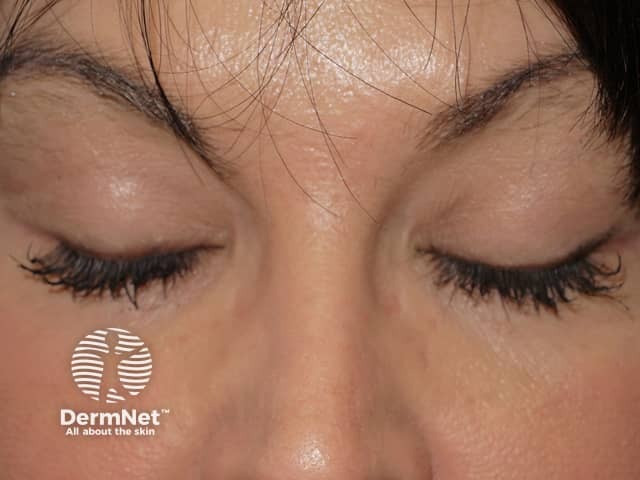
Effect of bimatoprost solution on eyelashes
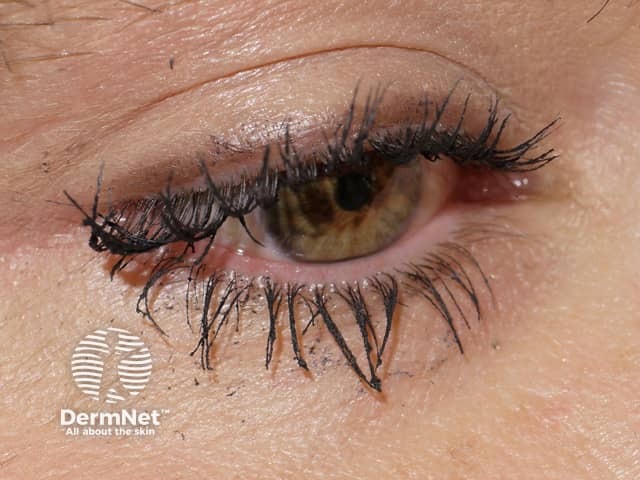
Effect of bimatoprost solution on eyelashes
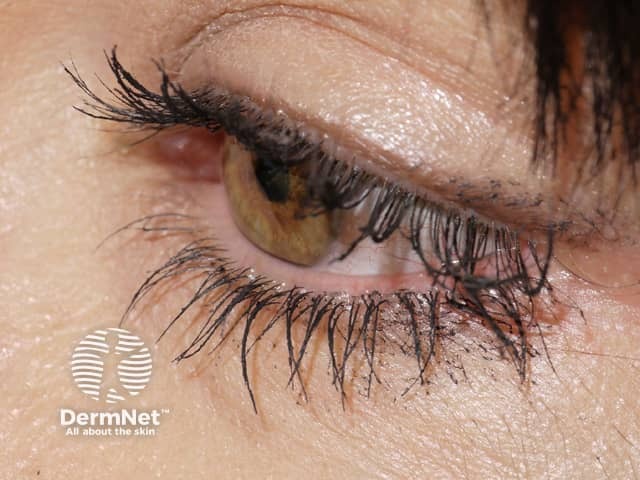
Effect of bimatoprost solution on eyelashes
The most frequently reported adverse events (< 4% patients) with bimatoprost solution are:
The following reactions have been identified during postmarketing use of bimatoprost solution in clinical practice:
There are no adequate and well-controlled studies of the use of bimatoprost ophthalmic solution 0.03% in pregnant women. Because animal reproductive studies are not always predictive of human response, bimatoprost solution should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether bimatoprost solution is excreted in human milk. Caution should be exercised when bimatoprost is administered to a nursing woman.
Safety and effectiveness in children have not been established.
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
In patients with a history of liver disease or elevated liver enzymes, bimatoprost solution 0.03% had no adverse effect on liver function over a period of 48 months.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).