Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence for sonidegib — extra information
Key clinical-trial evidence for sonidegib
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand; Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, August 2016.
Introduction Clinical trial experience Adverse reactions Long-term follow-up Future directions
Introduction
In July 2015 the US FDA (Food and Drug Administration) approved sonidegib (Odomzo®; Novartis, New Jersey, USA) for the treatment of adult patients with locally advanced basal cell carcinoma (BCC).
The European Commission approved the use of sonidegib for basal cell carcinoma in August 2015. In June 2016, sonidegib was granted marketing authorisation in Switzerland for treatment of adults with advanced basal cell carcinoma. The drug is also approved in Australia but is not available in New Zealand as yet.
Sonidegib is an inhibitor of the Hedgehog pathway. It binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction, which plays a critical role in stem cell maintenance and tissue repair.
Sonidegib is indicated for the treatment of adult patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy.
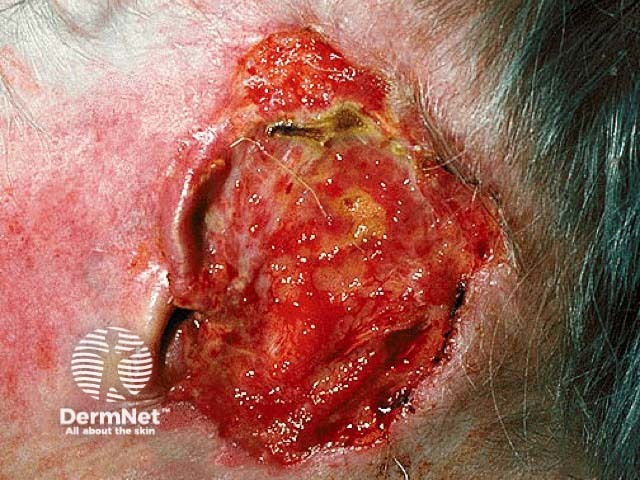
Advanced basal cell carcinoma
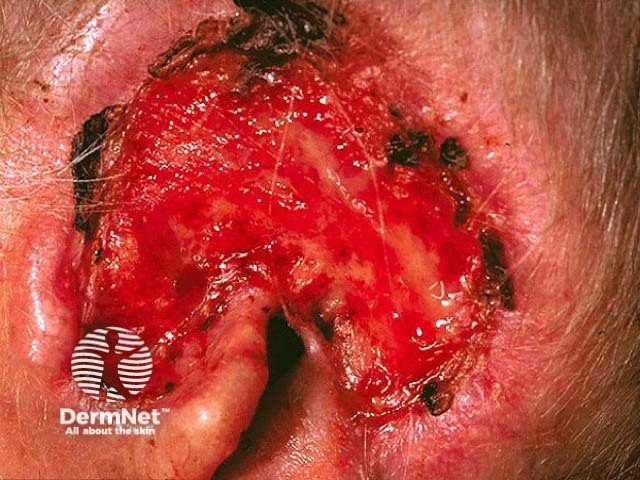
Advanced basal cell carcinoma
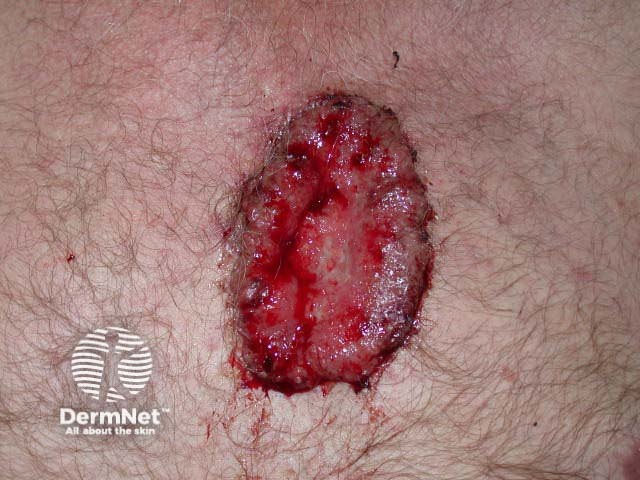
Advanced basal cell carcinoma
Clinical trial experience with sonidegib
- FDA approval was based on pivotal phase II study (BOLT trial) an international, multi-center, double-blind, randomised, two-arm, non-comparative trial in patients with locally advanced BCC not amenable to local therapy or metastatic basal cell carcinoma.
- Patients were randomised (2:1) to receive either sonidigib 800 mg (n = 151) or 200 mg (n= 79) orally, once daily, until disease progression or intolerable toxicity.
- Randomisation was stratified by stage of disease (locally advanced or metastatic), locally advanced BCC disease histology (aggressive versus non-aggressive), and geographic region.
- Patients with locally advanced BCC treated with sonidegib 200 mg (n=66) were followed for at least 12 months unless discontinued earlier.
- The objective response rate was 58% (95% confidence interval: 45-70) consisting of 5% (n=3) complete responses and 53% (n=35) partial responses.
- A pre-specified sensitivity analysis using an alternative definition for complete response, defined as at least a partial response according to MRI and/or photography and no evidence of tumour on biopsy of residual lesion, yielded a complete repsponse rate of 20%.
- Among the 38 patients with an objective response, 31 patients (82%) have ongoing responses ranging from at least 1.9 to 18.6 months.
- The median duration of response has not been reached.
- There was no evidence of better objective response rate among patients with locally advanced BCC randomised to receive sonidegib 800 mg daily and followed for at least 12 months.
- Chemotherapy included either dacarbazine 1000 mg/m2 every 3 weeks or the combination of carboplatin AUC (area under curve) 6 every 3 weeks plus paclitaxel 175 mg/m2 every 3 weeks.
- The primary endpoint was objective response rate (ORR) defined as the proportion of patients with confirmed complete or partial tumour response, or shrinkage, as measured by a central review committee.
- The evaluation of tumour response was based on a composite assessment that integrated tumour measurements obtained by radiographic assessments of target lesions (per RECIST 1.1 or mRECIST), digital clinical photography, and histopathology assessments (via punch biopsies).
- All modalities used must have demonstrated absence of tumour to achieve a composite assessment of complete response.
- Duration of response, determined by blinded central review, was a key secondary outcome measure.
Adverse reactions: clinical trial experience
- Adverse reaction rates observed in the clinical trials of a drug may not reflect the rates observed in clinical practice because clinical trials are conducted under widely varying conditions.
- The frequency of common adverse was greater in patients treated with sonidegib 800 mg compared to 200 mg.
- Tables 1 and 2 reflect adverse events in > 10% of patients exposed to sonidegib 200 mg daily (79 patients with locally advanced BCC [n=66] or metastatic BCC [13]).
- Patients were followed for at least 18 months.
- The median duration of treatment was 11.0 months (range 1.3 to 33.5 months).
Musculoskeletal and connective tissue disorders
- Muscle spasms 54% (3% Grade 3)
- Myalgia (muscle pain) 19% (0)
- Musculoskeletal pain 32 (1%)
Skin and subcutaneous tissue disorders
Nervous system disorders
- Dysgeusia (abnormal taste) 46% (0 Grade 3)
- Headache 15% (1%)
Administration site reactions
- Pain 14% (1% Grade 3)
Gastrointestinal disorders
- Nausea 39% (1% Grade 3)
- Diarrhoea 32% (1%)
- Abdominal pain 18% (0)
- Vomiting 11% (1%)
Metabolism and nutrition
- Decreased appetite 23% (1% Grade 3)
Key laboratory abnormalities
- Increased serum creatine kinase (muscle enzyme) 61% (8% Grade 3–4)
- Hyperglycaemia 51% (4%)
- Increased alanine aminotransferase 43% (13%)
- Increased aspartate aminotransferase 19% (4%)
- Increased amylase 16% (1%)
- Anaemia 32% (0)
- Lymphopenia (reduced lymphocytes) 28% (3%)
Long-term follow-up — clinical trial experience
- Sonidegib showed continued antitumour activity with no new safety concerns in patients with advanced basal cell carcinoma, according to results from a 30-month follow-up of the pivotal BOLT trial presented at the 2016 ASCO Annual Meeting.
- The primary outcome measure was objective response rate.
- Secondary endpoints included duration of response, complete response, progression-free survival, overall survival and safety.
- At the 30-month follow-up, patients with locally advanced BCC had an objective response rate of 56% and 45% at the 2 study doses of 200 mg and 800 mg of sonidegib, respectively.
- Among patients with metastatic BCC, the objective response rates at the lower and higher doses were 8% and 17%, respectively.
- In the locally advanced group, the complete response rate was 5%, the partial response rate was 52%, and the stable disease rate was 35%.
- Median duration of response was 26.1 months, the median progression free survival was 22.1 months, and the 2-year overall survival rate was 93% (95% CI, 80-98).
- In patients with metastatic BCC treated with the 200-mg dose, there were no complete responses, the partial response rate was 8%, the stable disease rate 85%, and rate of progressive disease was 0.
- The median duration of response was 24 months, the median progression free survival was 13.1 months (95% CI, 5.6-33.1) and the 2-year overall survival rate was 69% (95% CI, 31-89).
- All patients receiving the 800-mg dose and 97.5% of patients receiving the 200-mg dose experienced at least 1 adverse event.
- The rates of grade 3/4 adverse events (43% versus 64%) and serious adverse events (20% versus 39%) were lower with the 200-mg versus the 800-mg dose.
- Among all 79 patients who received 200-mg of sonidegib, the most common all-grade adverse events were muscle spasms (54%), alopecia (49%), dysgeusia (44%), nausea (39%), diarrhoea (32%), and weight decrease (30%).
- The most common grade 3/4 adverse events were creatinine kinase increase (7%), weight decrease (5%), muscle spasms (3%).
Future directions for sonidegib
- Sonidegib has demonstrated efficacy in the treatment of unresectable locally advanced and metastatic basal cell carcinoma.
- The toxicities identified are manageable and reversible upon discontinuation of treatment.
- However, sonidegib has not yet been compared to other approved hedgehog pathway inhibitor treatments for basal cell carcinoma, such as vismodegib.
- According to results presented at the 2016 ASCO Annual Meeting, a large trial (STEVIE Trial; n = 1215) has confirmed the benefits of vismodegib for basal cell carcinoma.
- The acceptable benefit-risk profile of sonidegib, along with a paucity of treatment options makes sonidegib an emerging option for the treatment of adults with locally advanced BCC that has recurred following surgery or radiation therapy, or in those who are not candidates for surgery or radiation therapy.
- Continued approval of sonidegib for treatment of basal cell carcinoma will depend on verification of clinical benefit in confirmatory trials.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Chen L, Silapunt S, Migden MR. Sonidegib for the treatment of advanced basal cell carcinoma: a comprehensive review of sonidegib and the BOLT trial with 12-month update. Future Oncol 2016; 12: 2095–105. PubMed
- Dummer R, Guminski A, Gutzmer R, et al. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol 2016; 75: 113–125.e5. DOI: 10.1016/j.jaad.2016.02.1226. PubMed
- Danial C, Sarin KY, Oro AE, Chang AL. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res 2016; 22: 1325–9. PubMed Central
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol 2015; 16: 716–28. PubMed
On DermNet
Other websites
- Prescribing information (Sonidegib, US Label)
- Sonidegib — European Medicines Agency marketing Authorization
- Basal cell carcinoma treatment and management — Medscape
