Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author: Anoma Ranaweera B.V.Sc; PhD (Clinical Biochemistry, University of Liverpool, UK); Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, August 2016.
Introduction
Uses
How it works
Dosage and administration
Potential drug interactions
Adverse effects
Warnings and precautions
In July 2015 the US FDA (Food and Drug Administration) approved sonidegib (Odomzo®; Novartis, New Jersey, USA) for the treatment of adult patients with locally advanced basal cell carcinoma (BCC).
The European Commission approved the use of sonidegib for basal cell carcinoma in August 2015. In June 2016, sonidegib was granted marketing authorisation in Switzerland for treatment of adults with advanced basal cell carcinoma. The drug is also approved in Australia. Additional regulatory submissions are being reviewed by health authorities worldwide.
Sonidegib is indicated for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy.
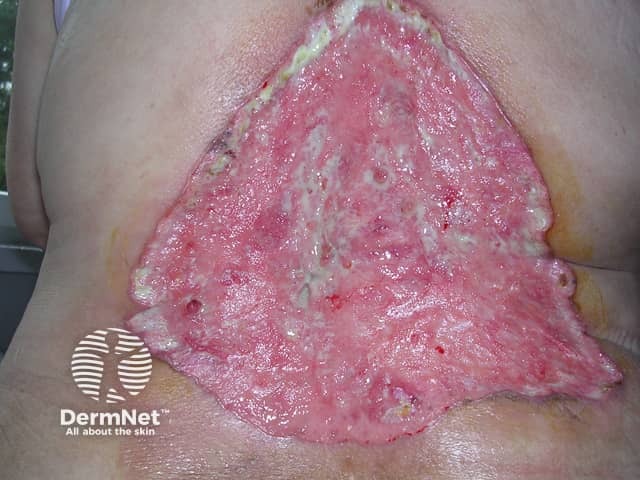
Advanced basal cell carcinoma
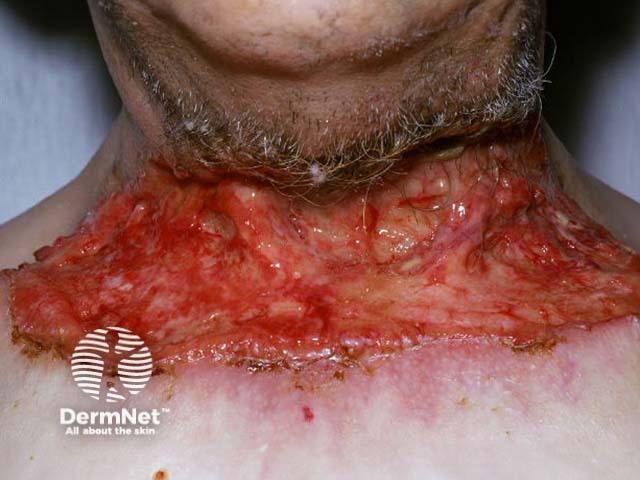
Advanced basal cell carcinoma
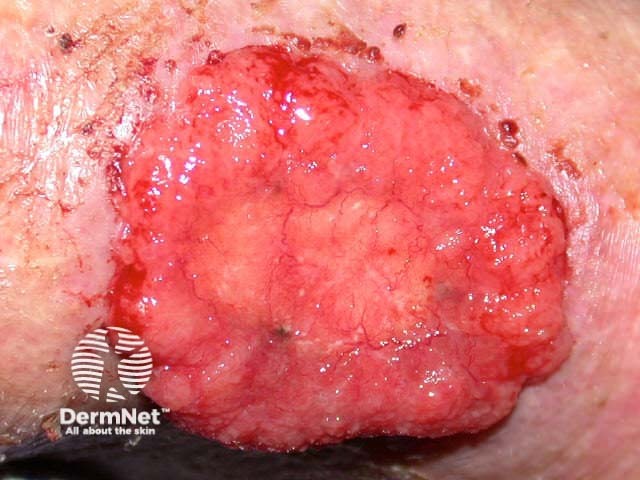
Advanced basal cell carcinoma
Link to key clinical-trial evidence about sonidegib
The most common (≥ 10%) adverse reactions associated with sonidegib treatment in clinical trials were:
The safety and effectiveness of sonidegib have not been established in children (< 18 years of age).
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).