Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author: DermNet Editor in Chief Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. January 2018.
This article was supported by an educational grant from Roche New Zealand, distributors of Cotellic™ (cobimetinib) in New Zealand. Sponsorship does not influence content.
Introduction
How they work
Demographics
Types of targeted therapies
Side effects
Efficacy
Targeted cancer therapies are drugs used to treat certain malignant tumours by blocking the action of particular molecular targets such as genes and proteins. They are classified as small molecules or monoclonal antibodies.
They are also called precision medicines.
Targeted cancer therapies interact with a target molecule to prevent tumour cells proliferating.
Targeted cancer therapies are classified as:
Targeted therapies are currently used in dermatology for patients with advanced cancers. They are also used in clinical trials as adjuvant therapy (supplementary treatment given to people who are in remission from their cancer but are at high risk of relapse in the future).
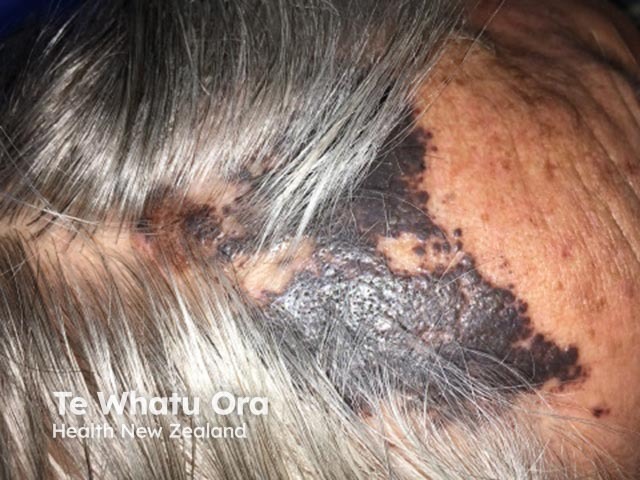
Satellite melanoma metastases
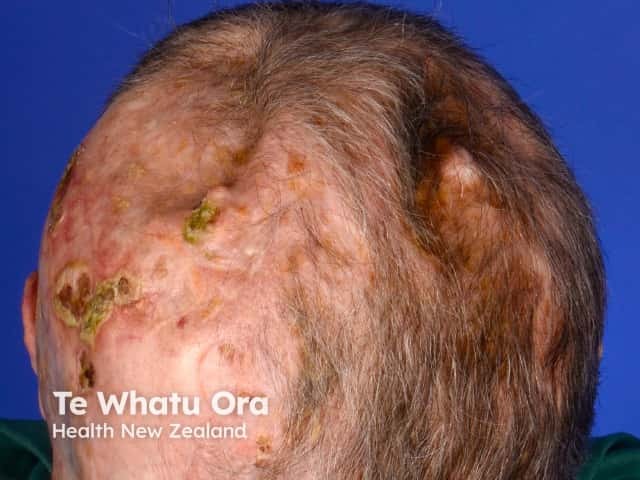
Scarring from multiple basal cell carcinomas
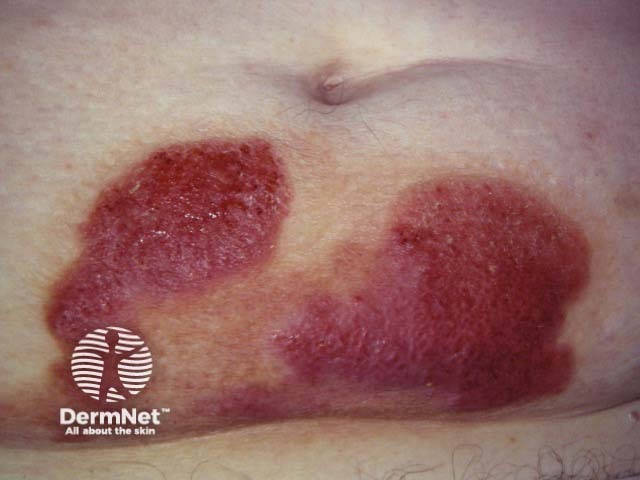
Cutaneous lymphoma
Several targeted therapies are marketed in dermatology to treat advanced skin cancers, and others are in development.
Several products are marketed to treat unresectable metastatic melanoma.
Vismodegib and sonidegib are available treatments for advanced basal cell carcinoma. They target Smoothened, a protein involved in the abnormal Hedgehog signalling pathway.
Several products are being investigated to treat advanced cutaneous squamous cell carcinoma; these include:
Targeted treatments used in refractory cutaneous T-cell lymphoma and Kaposi sarcoma are based on the retinoid pathway.
Rituximab is used in cutaneous B-cell lymphoma. Rituximab targets the CD20 antigen found on B cells.
Imatinib is used as a treatment in dermatofibrosarcoma protuberans. Imatinib targets BCR-ABL fusion protein.
Targeted therapies have many side effects, including adverse cutaneous reactions. In some cases, side effects are associated with excellent tumour response.
See Cutaneous adverse effects of checkpoint inhibitors and individual drug topics for more details.
The efficacy of targeted therapies is variable. They may lose their efficacy over time, having been initially successful at reducing tumour growth and in some cases leading to complete remission.
See individual drug topics for more details.