Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell/Maria McGivern. November 2018.
Introduction How it works How to use Potential drug interactions Adverse events Risks Uses in specific populations
Cemiplimab (also called cemiplimab-rwlc; trade name Libtayo®) is a prescription medicine used to treat people with cutaneous squamous cell carcinoma (SCC).
In April 2018, the European Medicines Agency (EMA) accepted for review the marketing authorisation application for cemiplimab for the treatment of patients with metastatic cutaneous SCC or locally advanced cutaneous SCC who are not candidates for surgery.
In September 2018, the US Food and Drug Administration (FDA) approved cemiplimab as a breakthrough therapy for the treatment of patients with metastatic cutaneous SCC or locally advanced SCC who are not candidates for curative surgery or curative radiation. Regeneron and Sanofi-Aventis (New Jersey, USA) will market Libtayo® jointly in the US.
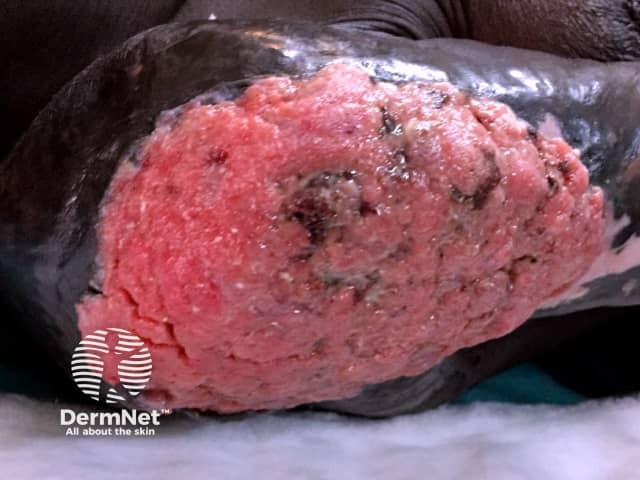
Advanced squamous cell carcinoma arising in thermal burn scar
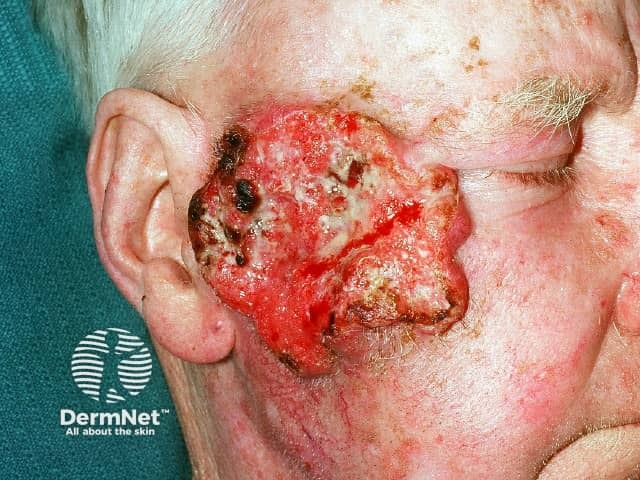
Advanced squamous cell carcinoma
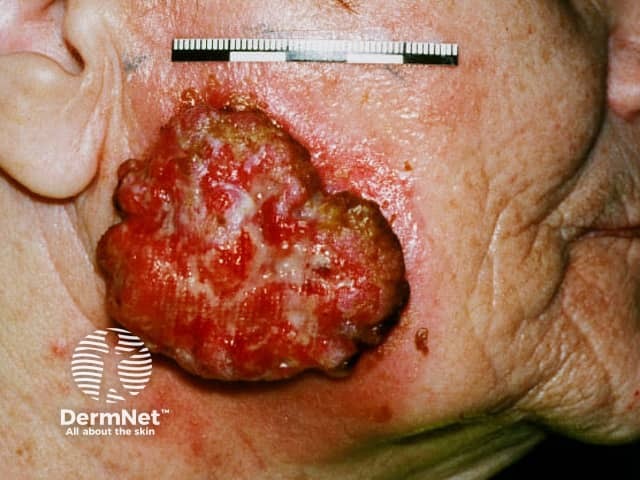
Advanced squamous cell carcinoma
Cemiplimab is the first and only treatment specifically approved and available for advanced cutaneous SCC in the US. This drug was evaluated by the FDA under priority review, which is reserved for medicines that represent significant improvements in safety or efficacy in treating serious conditions.
The EMA review process is anticipated to be complete in the first half of 2019. There are currently no EMA-approved treatments for advanced cutaneous SCC.
Cemiplimab is under investigation in other malignancies. It is in Phase III development for cervical cancer and basal cell carcinoma, and in Phase II development for other cancers including glioblastoma multiforme, ovarian and prostate cancers, and acute lymphocytic leukaemia.
Regulatory applications in additional countries are currently being considered for submission. Cemiplimab is currently not available in New Zealand.
Cemiplimab is a highly selective humanised monoclonal immunoglobulin G4 (IgG4) antibody that targets checkpoint inhibitor programmed cell death protein 1 (PD-1) and ligand 2 (PD-L2), releasing the PD-1 pathway-mediated inhibition of the immune response. This includes the anti-tumour immune response, thereby decreasing tumour growth.
The recommended dosage of cemiplimab is 350 mg as an intravenous infusion over 30 minutes every 3 weeks until disease progression decreases or unacceptable toxicity is reached.
Severe and fatal immune-mediated adverse reactions may necessitate the withholding or discontinuing of cemiplimab.
Cemiplimab should be withheld in the following conditions:
Cemiplimab should be permanently discontinued in the following conditions:
No dosage adjustment is necessary for mild or moderate renal impairment as no clinically important effect on the exposure of cemiplimab has been observed in pharmacokinetic population studies.
No dosage adjustment is necessary for mild hepatic impairment as no clinically important effect on the exposure of cemiplimab has been observed. There are no studies in patients with moderate or severe hepatic impairment.
No formal pharmacokinetic drug interaction studies have been conducted with cemiplimab.
In clinical trials, the most common adverse events (≥ 10%) in patients receiving cemiplimab up to 3 mg/kg every 2 weeks were:
The most frequent (≥2%) serious adverse drug reactions observed with cemiplimab were urinary tract infection, sepsis, pneumonia, pneumonitis, and cellulitis. Cemiplimab can also cause infusion reactions, including anaphylaxis.
Signs of these skin problems may include:
Before receiving treatment with cemiplimab, the healthcare provider should be informed if the patient has any of the following characteristics:
It is not known whether cemiplimab or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, mothers should discontinue nursing prior to using cemiplimab.
Females of reproductive potential should be advised to use effective contraception during treatment with cemiplimab and for at least 4 months after the last dose of the drug.
The safety and effectiveness of cemiplimab have not been established in children.
In clinical studies, of the 163 patients with metastatic and locally advanced cutaneous SCC who received cemiplimab, 72% were 65 years or older and 37% were 75 years or older. No overall differences in safety or effectiveness were observed between these and younger patients.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).