Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Elaine Luther, Medical Student, Ross University School of Medicine, Barbados, West Indies. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. May 2020.
Introduction
Uses
Contraindications
Names
Benefits
Disadvantages
Side effects and risks
In the strictest dictionary definition, a biological treatment or biologic drug would be any drug that is a biological product — this is true for most drugs, as they are extracted from plants, animals, and fungi, or developed through bioengineering.
In actual use, the terms 'biological treatment' and ‘biologic’ refers to bioengineered monoclonal antibodies. These are made by laboratory animals against a target antigen. The antibodies are ‘monoclonal’, meaning [1]:
Biological treatments are used to treat severe and refractory cases of inflammatory diseases, autoimmune diseases, and some cancers. Biological treatments are very effective, but because of their expense, they are typically reserved for patients who have failed at least three other treatments.
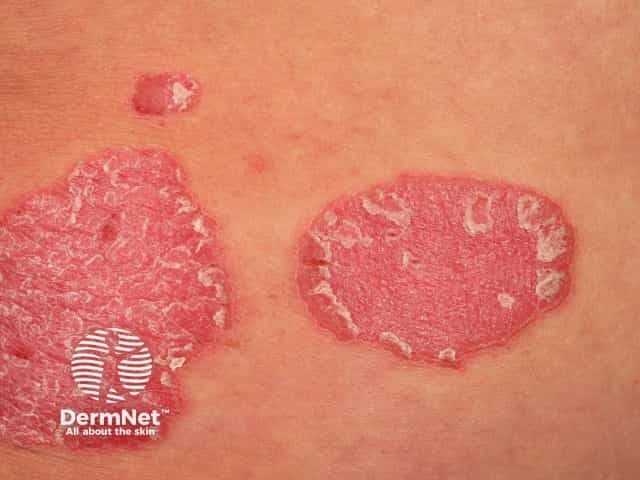
Chronic plaque psoriasis
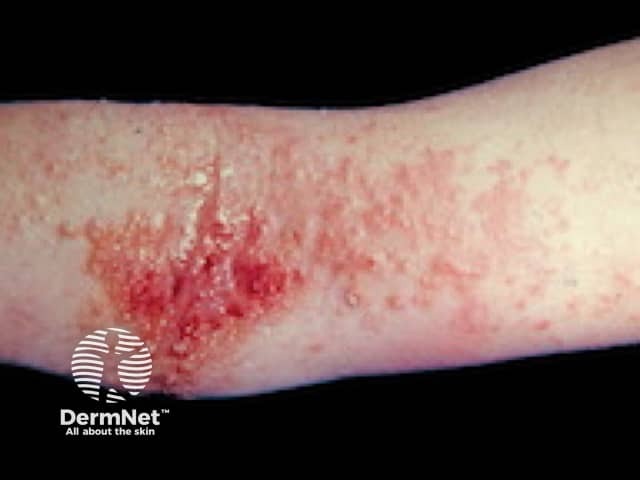
Atopic eczema
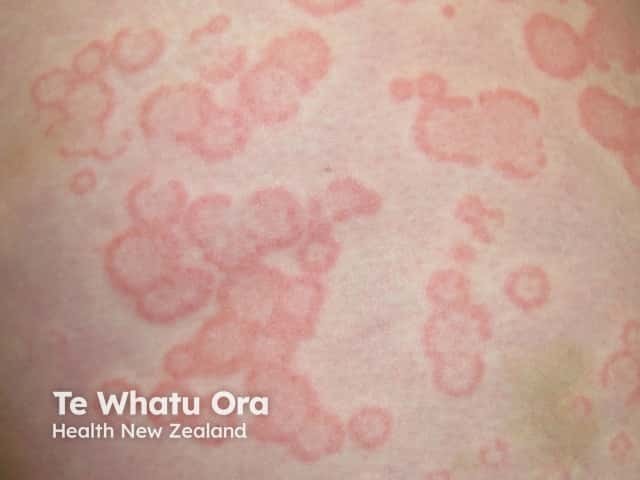
Urticaria
Contraindications to a biological treatment include:
The World Health Organization (WHO) dictates the names of generic drugs.
International Nonproprietary Names (INNs) for monoclonal antibodies shall be composed of a prefix that can be anything, a target (substem A), a species of origin (substem B), and a suffix (-mab) [2].
The prefix can be random; the only requirement is to contribute to a pleasant-sounding and distinctive name.
‘Substem A’ refers to the first of two internal stems that identify the type of drug. Substem A indicates the type of target antigen — a molecule, a cell, or an organ. A single letter is used when possible. If a vowel is also needed to aide pronunciation, use the vowel indicated in parentheses in Table 1.
Target antigen type |
Substem A |
|---|---|
Bacterial |
-b (a)- |
Cardiovascular |
-c (i)- |
Fungal |
-f (u)- |
Interleukin |
-k (i)- |
Immunomodulating |
-l (i)- |
Neural |
-n (e)- |
Bone |
-s (o)- |
Toxin |
-tox (a)- |
Tumour |
-t (u)- |
Viral |
-v (i)- |
With a single letter, Substem B describes the animal or human species or species (a chimera) on which the sequence of the monoclonal antibody is based.
The species |
Substem B |
|---|---|
Rat |
-a- |
Rat + mouse |
-axo- |
Hamster |
-e- |
Primate |
-i- |
Mouse |
-o- |
Human (fully human) |
-u- |
Chimeric |
-xi- |
Chimeric + humanised |
-xizu- |
Humanised |
-zu- |
A chimeric antibody is constructed from two sources: a foreign variable domain (ie, the V-D-J region) is synthesised or originates from a non-human species linked to a constant (Fc) region of human origin.
A humanised antibody is a chimeric antibody constructed using antibody engineering. It consists of synthetic or non-human complementarity-determining regions of the variable domains and the remaining chain is of human origin.
All monoclonal antibodies have the same suffix: -mab. This -mab suffix is used for all products containing an immunoglobulin variable domain that binds to a defined target.
Biological treatments are especially useful because they tweak only one part of the immune system, rather than suppressing the entire system. Biological treatments can often rapidly improve refractory skin conditions such as psoriasis, atopic eczema, and urticaria.
The disadvantages of biological treatments include:
Side effects and risks of biological treatments relate to suppression of components of the immune system and the induction of allergic reactions.
A biological treatment binds a single target on an immune cell, such as an overactive interleukin (IL) or a surface protein that has led to the disease undergoing treatment (for example, targeting IL-17, a cause of psoriasis). This binding deactivates the target (eg, 1L-17, improving the psoriasis), but also suppressing the normal immune function against certain infections (such as tuberculosis or candidiasis).
Prior to treatment with a biological treatment, patients must be screened for infections (such as tuberculosis, human immunodeficiency virus, and viral hepatitis) and should not have cancer. During treatment, they should be monitored with routine blood tests for infection markers and have cancer checks, including total body skin examinations.
Because biological treatments are large proteins pharmed from mice, allergic reactions can occur.
The side effects of each biological treatment are unique. Physicians should know the possible adverse events associated with a biological treatment before it is prescribed.