Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Harmony Thompson, Medical Registrar, Hamilton, New Zealand. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. June 2020.
Introduction
Immune adverse-events
Definition - cutaneous adverse effects
Differential diagnoses
Diagnosis
Treatment
Outcome
A checkpoint inhibitor is a form of immunotherapy used to treat cancer that targets the immune response. It enhances the immune system by inhibiting the ‘brake’ on an activated immune system (cytotoxic T-lymphocyte-associated antigen 4 [CTLA-4] inhibitors such as ipilimumab, or by blocking the immune inhibitory expression by the cancer cells (programmed cell death protein 1 [PD1] such as pembrolizumab and nivolumab, or PD-L1 inhibitors such as avelumab). Immune checkpoints are essential in maintaining immune homeostasis by augmenting or inhibiting immune responses and allowing tolerance to self-antigens [1–4].
Checkpoint inhibitors can cause a wide spectrum of side effects due to their triggering cytotoxic CD4+/CD8+ T-cell activation. More than 60% of patients develop immune-related adverse events, which can theoretically affect any organ [1–4].
Immune-related adverse events associated with checkpoint inhibitors include:
Other reactions include:
Adverse effects involving the skin are among the most common immune-related adverse events due to checkpoint inhibitors, occurring in 30–60% of patients. Various presentations are described below [5–8].
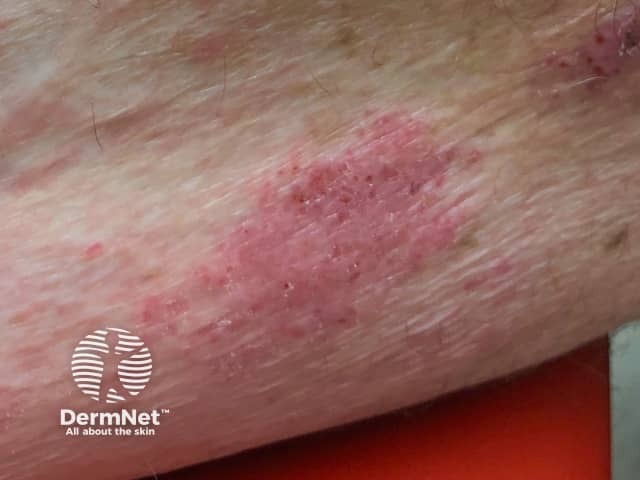
Dermatitis induced by pembrolizumab
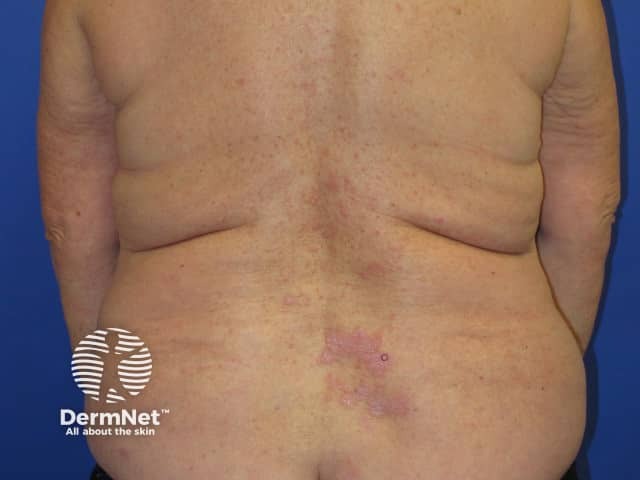
Nivolumab-induced lichenoid dermatitis
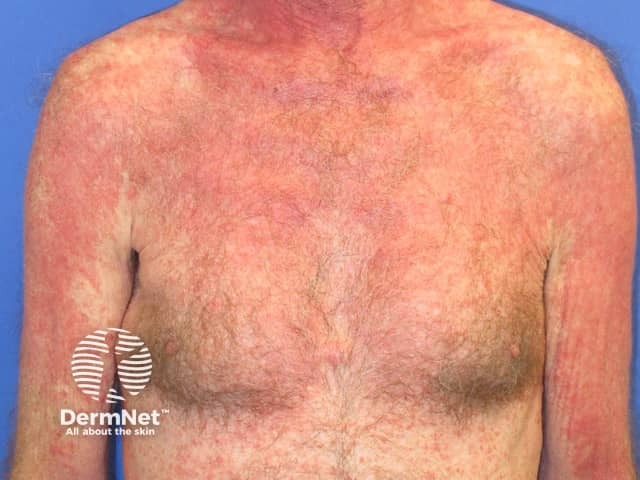
Pembrolizumab-induced psoriasis
Dermatitis often starts after the first few cycles of treatment, with worsening after each subsequent cycle of treatment. Delayed eruptions can also occur.
Lichenoid drug reactions start after several weeks to months of treatment.
Psoriasis develops several months after treatment.
See more on immunotherapy-related psoriasiform reactions.
Severe and life-threatening cutaneous reactions (SCARs) include:
Vitiligo can present as localised or widespread areas of depigmentation.
Pembrolizumab and nivolumab are being reported to trigger eosinophilic fasciitis.
Other skin diseases associated with checkpoint inhibitors include:
It can be difficult to distinguish between the adverse effects of immunotherapy and primary skin disease. It is important to rule out infection and the adverse cutaneous effects of other drugs [3].
The specific diagnosis may be clear by taking a history and performing a careful examination.
Possible investigations for patients with skin problems on checkpoint inhibitors include:
A skin biopsy may be helpful to differentiate various inflammatory rashes.
To decide on treatment, the clinical severity of a skin problem needs to be carefully assessed in conjunction with the impact on activities of daily living. Dermatologist input and skin biopsy should be obtained prior to commencing systemic corticosteroids (which are contraindicated for psoriasis and ineffective for vitiligo).
There are four grades of severity.
Symptoms in Grade 1 include:
To alleviate these symptoms, continue immunotherapy, treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider the use of mild to moderate-potency topical steroids.
Symptoms in Grade 2 include:
To alleviate these symptoms, consider interrupting immunotherapy until the rash has reverted to grade 1. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids, or systemic corticosteroids (eg, prednisone 0.5–1 mg/kg/day).
Symptoms in Grade 3 include:
Consider withholding immunotherapy and consult with dermatologist. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids, and systemic corticosteroids (eg, prednisone 0.5–1 mg/kg/day or IV methylprednisolone 1–2 mg/kg/day with slow tapering).
Symptoms in Grade 4 include:
It is recommended to immediately stop and permanently discontinue immunotherapy if Grade 4 symptoms are present, and a dermatologist should be consulted. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids. Consider IV methylprednisolone 1–2 mg/kg/day with slow tapering.
If immunotherapy should be suspended, then only resume when the steroid dose is < 10 mg/day of prednisone equivalent [1–8].
Other possible treatments for any dermatosis secondary to checkpoint inhibitors can include:
A multidisciplinary approach is necessary, with close communication between oncologists, dermatologists, and the patient. The treatment of immune-related immune reactions does not appear to affect the cancer response to checkpoint inhibitors. Note that prolonged immunosuppressive treatment increases the risk of opportunistic infections.
Most cutaneous adverse reactions to checkpoint inhibitors are mild (Grade 1) and resolve with topical treatment. SCARs are rare but can be life-threatening and may require admission to an intensive care unit.
Rashes due to immunotherapy can often take a long time to resolve and can persist for months after immunotherapy is discontinued.