Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Reactions Autoimmune/autoinflammatory
Last Reviewed: January, 2024
Author(s): Dr Riyad N.H. Seervai, Dermatology Resident, Oregon Health and Science University; Sarah K. Friske, Baylor College of Medicine, Texas USA (2024)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Demographics
Causes
Clinical features
Clinical features in varying skin types
Complications
Diagnosis
Differential diagnosis
Treatment
Outcome
Immunotherapy-related psoriasis is a subset of psoriasis or psoriasiform immune-related adverse events (irAEs) seen in patients treated with immune checkpoint inhibitor anticancer therapy. These reactions comprise ~4% of all skin toxicities associated with immunotherapy.
Most psoriasiform irAEs are mild, involving 10% body surface area (BSA). However, 10.5% of patients develop severe reactions involving >30% BSA and 2.5% develop erythroderma.
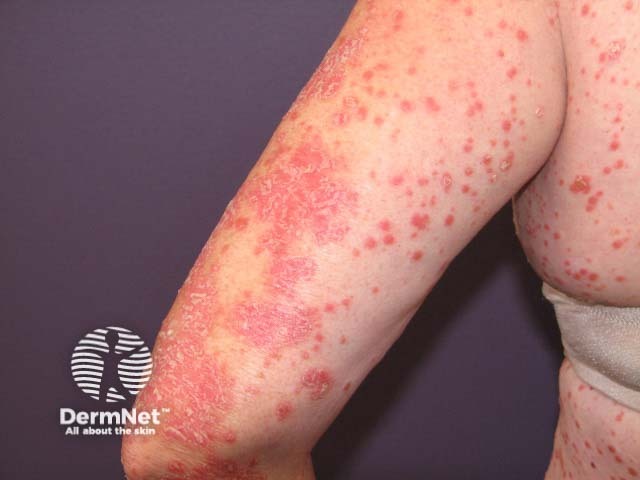
Psoriasifom eruption induced by pembrolizumab
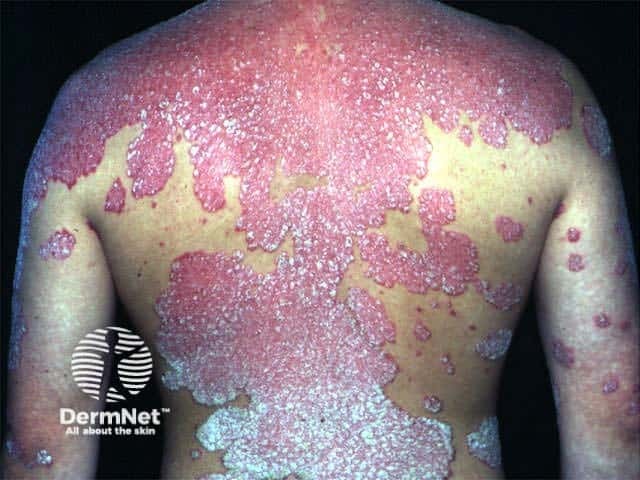
Psoriasis has extended in this patient with pre-existing disease after starting nivolumab
Demographic characteristics favour:
A personal history of psoriasis and a family history of psoriasis have also been linked to the development of immunotherapy-related psoriasis in about one-third of cases.
Active psoriasis at baseline is associated with a more rapid deterioration of the disease following immune checkpoint inhibitor (ICI) introduction.
Psoriasis onset or exacerbation followed an average of 11.2 drug doses in a multicentre study of 115 patients, and was observed to occur after a higher mean number of infusions in women than men (14.9 vs 10.1 respectively, p = 0.025).
The pathogenesis of immunotherapy-related psoriasis is thought to be related to the alteration of the T helper cell Th1 and Th17 pathways caused by PD-L1 (programmed cell death ligand 1) inhibition and subsequent heightened expression of IL-17 (interleukin 17).
Increased infiltration of memory CD8+ T cells and increased expression of IL-6 (interleukin 6) are also postulated to play a role, as demonstrated by mouse models of psoriasiform immune-related adverse events with PD-L1 disruption.
The gold standard method to determine severity of psoriasis lesions and to evaluate treatment efficacy is the Psoriasis Area and Severity Index (PASI). However, one parameter assessed in PASI is erythema which is assessed visually and prone to inconsistent, subjective results. As skin types differ in their presentation of erythema, careful consideration of the limitations of current methods used to determine extent of disease is recommended in skin of darker pigmentation.
Arthritis has been reported as a complication of various types of psoriasis that develop following immunotherapy administration.
In addition, erythrodermic psoriasis, a rare and severe variant of psoriasis, can develop in patients treated with immune checkpoint inhibitor anticancer therapy. Complications specific to erythrodermic psoriasis include:
The diagnosis of immunotherapy-related psoriasis is often made clinically, and may be assisted by biopsy.
Histopathologic evidence for diagnosis includes:
Topical therapies with steroids and/or calcipotriol is the first-line treatment for immunotherapy-related psoriasis.
Systemic steroid use is common, however, it can lead to rebound disease on taper or discontinuation and is not recommended.
Multiple new biologic agents have been used to treat immunotherapy-related psoriasis:
Approximately half of patients who develop immunotherapy-related psoriasis require discontinuation, or at least interruption, of their immunotherapy treatment. Significant risk factors associated with eventual treatment modification include development of pustular psoriasis or disease involving more than 10% of body surface area. However, optimal management of immunotherapy-related psoriasis is crucial as severity of psoriasiform irAEs is a positive predictor of immune checkpoint inhibitor anti-tumour effect.