Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author: essence the health agency, Auckland, New Zealand; Editor-in-Chief: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, December 2018.
This article was supported by an educational grant from Novartis; distributers of Cosentyx® in New Zealand. Sponsorship doesn’t influence content.
Introduction
How it works
Efficacy in psoriasis
Tolerance
Administration
Precautons
Use in special patient groups
Use in pregnancy
Monitoring
Secukinumab belongs to the class of biological treatments known as monoclonal antibodies. The trade name for secukinumab is Cosentyx® (Novartis AG) [1].
Secukinumab is licensed for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy (a drug that is absorbed into the bloodstream and distributed to all parts of the body, eg, methotrexate, acitretin or ciclosporin) or phototherapy (light therapy). It is also approved for the treatment of psoriatic arthritis and ankylosing spondylitis [1].
Secukinumab was approved by the US Food and Drug Administration (FDA) in January 2015, and the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorisation for secukinumab.
In New Zealand, secukinumab was classified as prescription medicine in August 2015. Secukinumab has been funded in New Zealand as a first- and second-line treatment for severe chronic plaque psoriasis on Special Authority application since October 2018 [2].
Interleukin-17A (IL-17A) is a cornerstone cytokine (messenger protein) involved in the development of psoriasis and is found in high concentrations in psoriasis plaques [3]. See Interleukin-17 in inflammatory skin disorders.
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to IL-17A, rapidly inhibiting its pro-inflammatory effects [3,4].
A wealth of data supports the effectiveness of secukinumab in reducing disease activity (PASI score) in patients with psoriasis, with a fast onset of action and a favourable safety profile [1, 5–8].
Secukinumab has been shown to act rapidly to effectively relieve the symptoms of psoriasis [5–7]. The ERASURE study (N=738) reported a 50% reduction in mean PASI score as early as week 3 [5]. The CLEAR study (N=676) showed that 5/10 patients achieved 75% reductions in PASI score by week 4, which increased to 8/10 patients achieving a 90% reduction in the severity and extent of their psoriasis after 16 weeks of treatment [6, 7].
The efficacy of secukinumab is also maintained over the long-term – more than 9/10 patients maintained their PASI response for up to 5 years in the SCULPTURE extension study (N=168) [8].
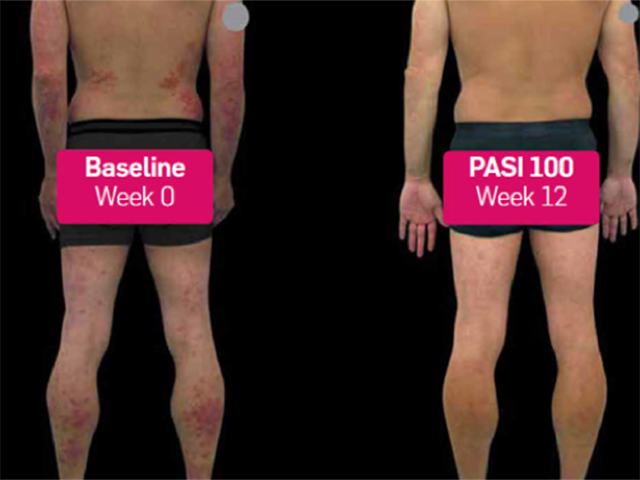
Before and after secukinumab
Secukinumab has a well-tolerated safety profile, with low reported immunogenicity [1].
Less than 1% of patients experience injection-site reactions. In clinical trials, the most frequently reported adverse events (> 2%) were upper respiratory tract infections (most frequently nasopharyngitis (11.4%) and rhinitis (2.5%)) and diarrhoea (4.1%). Most of the events were mild or moderate in severity [1].
If you experience any of the following signs or symptoms, you should contact your doctor [9].
Secukinumab has an established safety profile; no new safety signals were seen in patients treated with secukinumab for up to 5 years in the SCULPTURE extension study [8].
Secukinumab has demonstrated a comparable safety profile to ustekinumab and etanercept [5, 6].
Read more about key clinical-trial evidence with secukinumab.
Secukinumab is given by subcutaneous injection. The usual dose for psoriasis is 300 mg at Weeks 0, 1, 2, 3 and 4, followed by 300 mg every 4 weeks. Each 300 mg dose is given as two 150 mg injections [1].
After initial counselling and training by a healthcare professional, secukinumab can be self-administered by patients into the thigh or abdomen using a pre-prepared SensoReady pen, or by their carer into the upper arms. Each SensoReady pen contains a 150 mg dose of secukinumab. A different site should be used for each set of injections to reduce soreness and to prevent the skin from becoming tender, red, bruised or hard [1].
Secukinumab should be kept refrigerated at 2–8 °C until shortly before injection [1].
Secukinumab can be given alone or if necessary, in combination with methotrexate [1].
Secukinumab has the potential to increase the risk of infections, and so caution should be exercised when considering the use of secukinumab in patients with a chronic infection, or a history of recurrent infection [1].
In psoriasis clinical studies, the majority of infections observed in patients receiving secukinumab were mild or moderate in intensity. Non-serious Candida infections were more frequently reported in patients treated with secukinumab (1.2%) versus placebo (0.3%) [1].
Patients should be instructed to seek medical advice if signs or symptoms suggestive of an infection occur. If a patient develops a serious infection, the patient should be closely monitored and secukinumab should not be administered until the infection resolves [1].
Secukinumab is not associated with increased susceptibility to tuberculosis (TB). Patients should be evaluated for TB prior to initiation with secukinumab, and secukinumab should not be given to patients with active TB [1].
Caution should be exercised when prescribing secukinumab to patients with active Crohn disease, as exacerbations have been observed in clinical trials with secukinumab. Patients with active Crohn disease should be monitored closely when on treatment with secukinumab [1].
If anaphylaxis or another serious allergic reaction occurs, secukinumab should be discontinued immediately and appropriate therapy initiated. The SensoReady pen has a latex rubber stopper. Although no natural rubber latex is detected in the cap, the safe use of the SensoReady pen in latex‐sensitive individuals has not been studied [1].
Patients treated with secukinumab should not receive live vaccinations during treatment [1].
Therapeutic monitoring should be considered in patients being treated with concomitant CYP450 substrates with a narrow therapeutic index [1].
No dosage adjustment is needed for elderly patients (≥ 65 years of age). In pharmacokinetic studies, clearance of secukinumab was similar between elderly patients and those < 65 years of age [1].
Secukinumab has not been specifically studied in patients with renal or hepatic impairment, and so no dose recommendations can be made [1].
The safety and effectiveness of secukinumab in paediatric patients below 18 years of age has not yet been established [1].
There are no adequate data for the use of secukinumab in pregnant women [1].
Developmental toxicity studies conducted with monkeys found no evidence of harm to the fetus due to secukinumab [1].
Secukinumab should only be used in pregnancy if the benefits clearly outweigh the potential risks [1].
If secukinumab has been used during pregnancy, administration of live vaccines to newborns/infants for 16 weeks after the mother’s last dose of secukinumab is generally not recommended [1].
Caution should be exercised in women who are breastfeeding, as it is not known whether secukinumab or its metabolites are present in human milk [1].
It is recommended that patients on biologic medications have routine blood tests every 6 months or so, including full blood count and liver function tests.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).