Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Staff writer. Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, March 2015.
Introduction
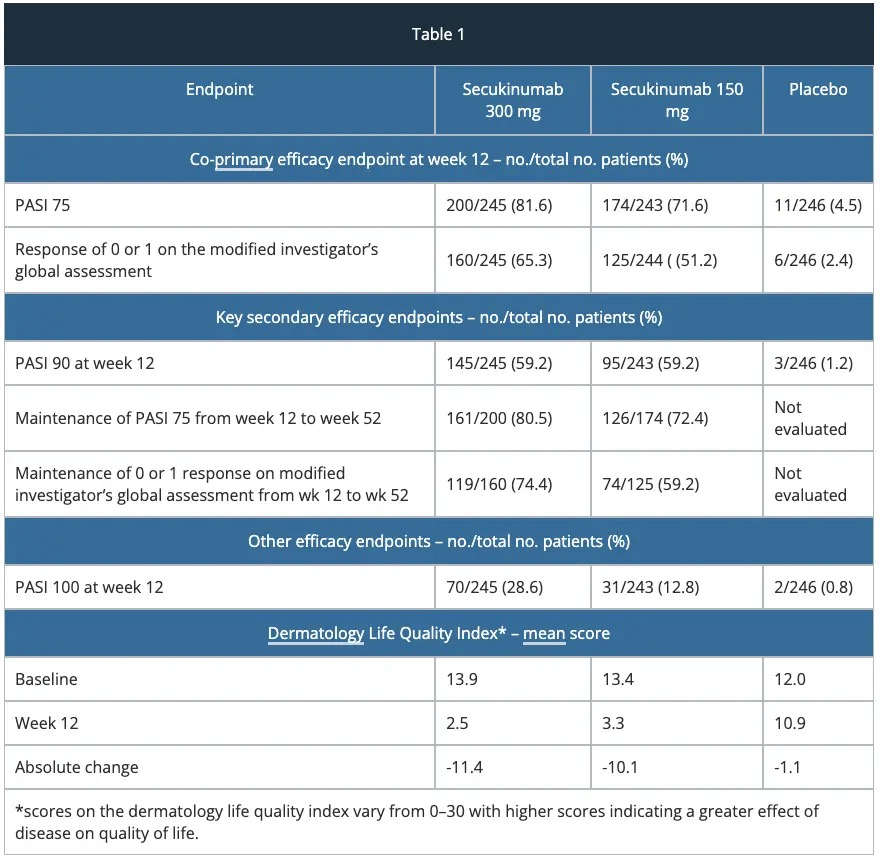
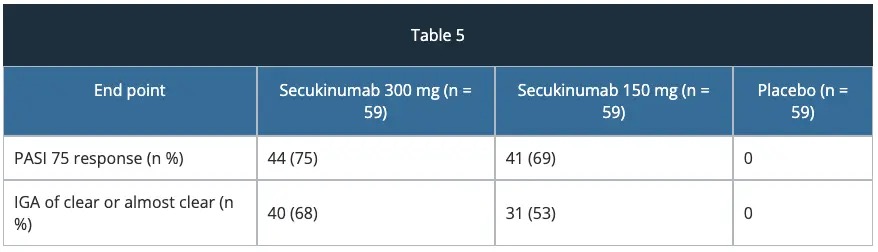
ERASURE study – efficacy
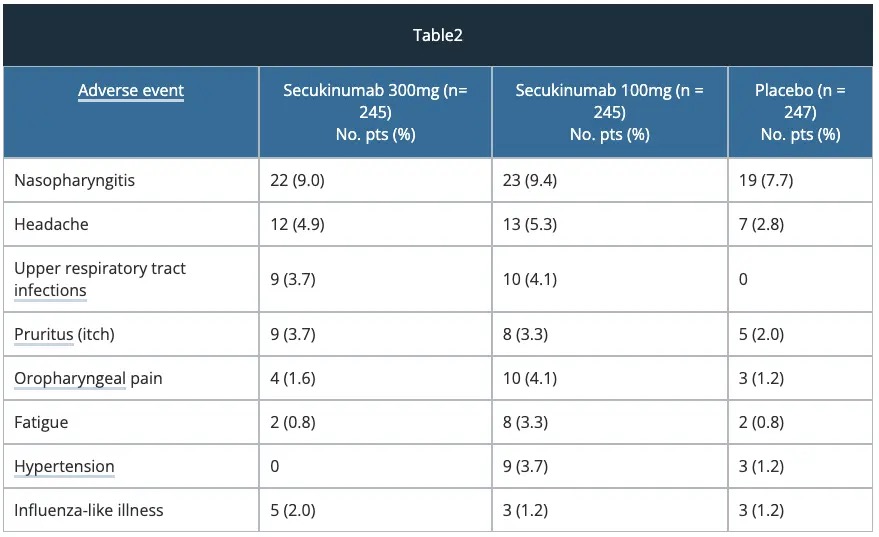
Safety — ERASURE study
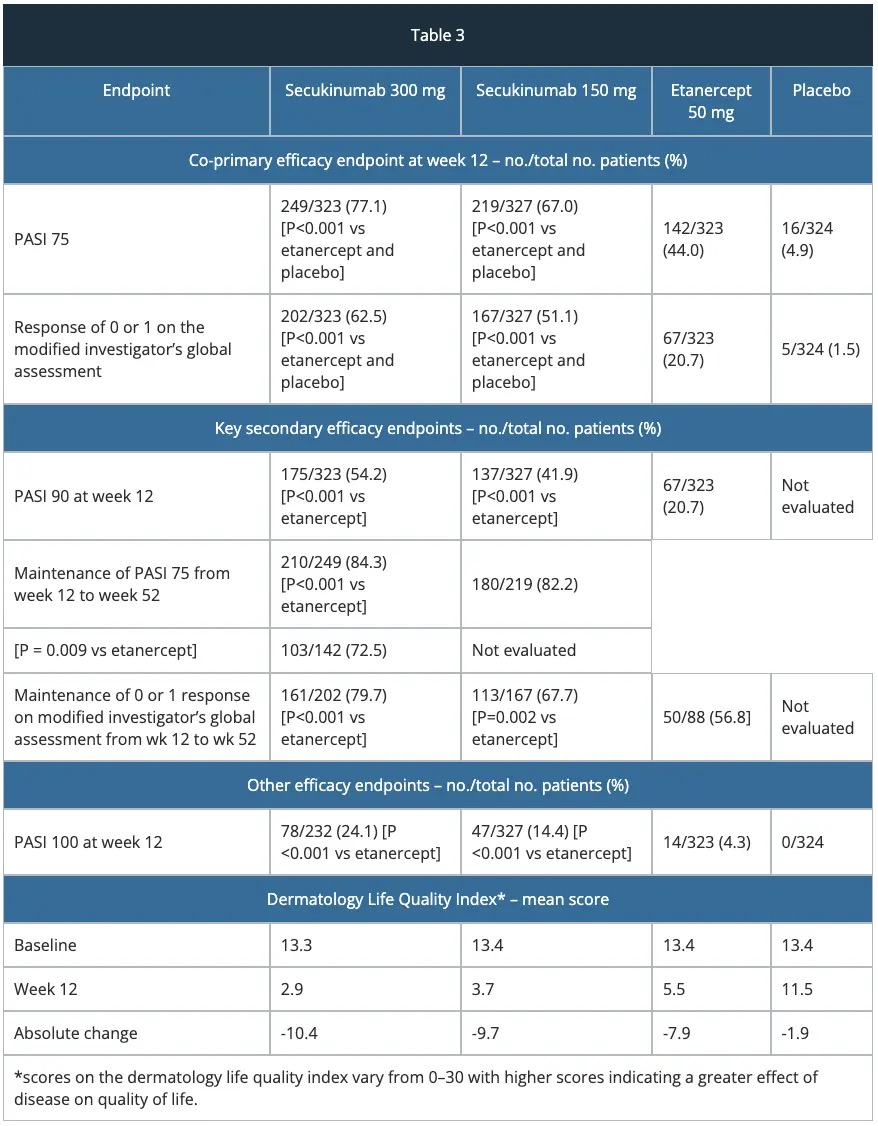
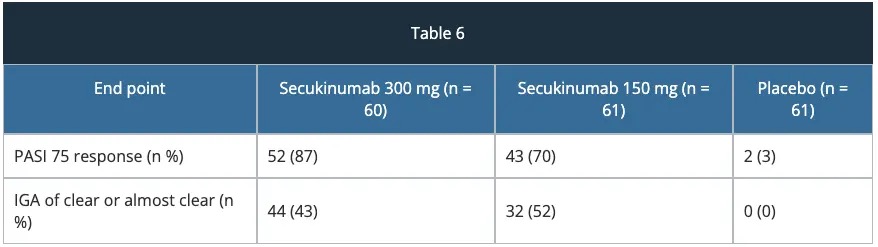
FIXTURE study – efficacy
Safety – FIXTURE study
FEATURE study
JUNCTURE study
Efficacy of secukinumab — real-world analysis
Guselkumab vs secukinumab — ECLIPSE study
Ustekinumab vs secukinumab - CLEAR study — long term sustained efficacy
Secukinumab — efficacy in palmoplantar psoriasis
Secukinumab — efficacy in scalp psoriasis
Secukinumab — efficacy in nail psoriasis
Future directions
In January 2015, the US Food and Drug Administration (FDA) approved secukinumab (Cosentyx™, Novartis, USA) for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorisation for secukinumab.
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the cytokine interleukin-17A (IL-17A) inhibiting its pro-inflammatory effects.
IL-17A is a key cytokine (messenger protein) involved in the development of plaque psoriasis and is found in high concentrations in psoriasis plaques.
The approval of secukinumab is based on the efficacy and safety outcomes from 10 Phase II and III studies which included over 3,990 patients with moderate-to-severe plaque psoriasis. These trials included four pivotal Phase III trials, ERASURE, FIXTURE, FEATURE and JUNCTURE studies detailed below.
The most common adverse reactions (occurring in > 2% of patients) at week 12 are summarised in table 4.
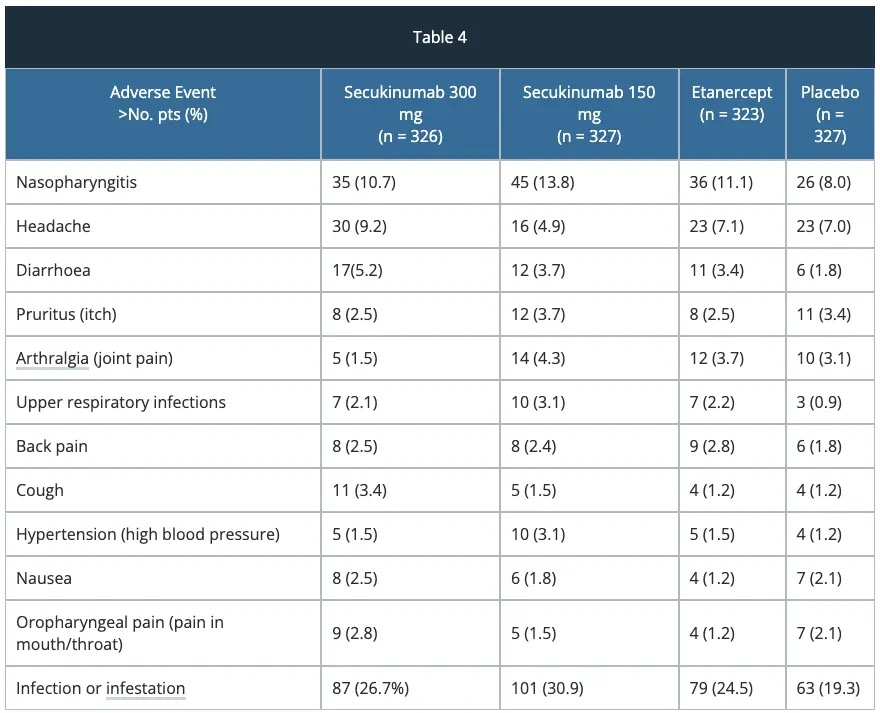
Adverse reactions that occurred at a higher incidence (> 2%) in secukinumab-treated patients vs placebo through to week 12 included nasopharyngitis, diarrhoea and upper respiratory tract infections.
Adverse reactions that occurred at a higher incidence (> 2%) in secukinumab-treated patients vs placebo through to week 12 included nasopharyngitis, diarrhoea and upper respiratory tract infections.
Non-trial data are needed in the efficacy and safety of secukinumab in routine clinical practice as most trial protocols prohibit concomitant psoriasis medication and specify transition periods.
The effectiveness and safety of secukinumab in the context of previous and concomitant treatments was assessed in the PROSPECT study [4]. This study is an ongoing 24-week single cohort non-interventional study which included 1988 patients with moderate to severe psoriasis receiving secukinumab 300 mg.
Mean baseline Psoriasis Area and Severity Index (PASI) score was 17.7±12.5. 90.9% of subjects had prior systemic treatment. Concomitant treatment was recorded in 44.3% of subjects. The median duration of the transition period was 14.0, 30.0, and 44.5 days from prior topical, conventional systemic, and biologic treatments. At Week 24 PASI75/90/100 was reached by 86.1%, 68.5%, and 39.7% of subjects who started secukinumab treatment at baseline. No unexpected safety signals were observed.
The safety and effectiveness of secukinumab were similar to that observed in the phase III clinical trial programme.
In this phase 3, multicentre, double-blind, randomised, comparator-controlled trial [5] at 142 outpatient clinical sites in nine countries, eligible patients were randomly assigned to receive either guselkumab (100 mg at weeks 0 and 4 then every 8 weeks; n =534) or secukinumab (300 mg at weeks 0, 1, 2, 3, and 4, and then every 4 weeks; n = 514).
The primary objective of this study was to show the superiority of clinical response at week 48 for guselkumab versus secukinumab.
The proportion of patients with a PASI 90 response at week 48 was greater in the guselkumab group (451 [84%]) than in the secukinumab group (360 [70%]; p < 0·0001).
However, no superiority was shown in favour of secukinumab for the major secondary endpoint of PASI 75 at both weeks 12 and 48.
Proportions of patients with adverse events, infections, and serious adverse events were similar between the two treatments and, in general, safety findings were consistent with registrational trial observations.
The CLEAR study [6] was a phase 3b, head-to-head, randomised, double-blind study on the efficacy and safety of secukinumab compared with ustekinumab over 52 weeks of treatment in adult patients with moderate-to-severe psoriasis.
Among 676 randomised subjects, secukinumab demonstrated superiority to ustekinumab at week 52 in the proportion of subjects with ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90) (76% vs 61% [P < .0001]); PASI 100 responses were 46% versus 36% (P = .0103) and Investigator's Global Assessment responses of clear/almost clear skin were 80% versus 65% (P < .0001). Subjects on secukinumab reported greater reductions in psoriasis-related pain, itching, and scaling, and greater improvement across all quality-of-life measures evaluated (Dermatology Life Quality Index (DLQI), EuroQoL 5-Dimension Health Questionnaire, Work Productivity and Activity Impairment Questionnaire-Psoriasis, and Health Assessment Questionnaire-Disability Index). The safety profile of secukinumab was comparable to that of ustekinumab.
In the long term sustained efficacy study, patients from the secukinumab arm who completed 52 weeks of treatment and consented to continue in an open-label extension phase received secukinumab 300 mg at week 52, followed by dosing every 4 weeks to week 100.
303 patients entered the extension phase and 277 patients completed the 2-year extension study.
Psoriasis Area and Severity Index 75 (89.6%), 90 (74.7%), and 100 (47.4%), and Investigator's Global Assessment 2011 modified version 0/1 response rates (68.8%) with secukinumab were sustained up to year 2. A similar trend was seen for the Dermatology Life Quality Index 0/1 response and the mean scores for patient assessment of psoriasis-related pain, itching, and scaling severity up to year 2 of secukinumab treatment. A high proportion of patients achieved complete relief (score 0) of psoriasis-related pain, itching, and scaling sustained up to year 2.
The 52-week results from the CLEAR study [6] showed high and superior efficacy of secukinumab versus ustekinumab in clearing skin and improving patient-reported outcomes, with a comparable safety profile in subjects with moderate to severe psoriasis.
Similar to the core study, secukinumab showed sustained and superior efficacy with faster response versus ustekinumab, and no new or unexpected safety concerns in Asian subjects with moderate to severe plaque psoriasis [7].
The GESTURE study [8] investigated the long-term (2·5 year) safety and efficacy of subcutaneous secukinumab 150 and 300 mg in 205 subjects with moderate-to-severe palmoplantar psoriasis (ppPsO).
The primary endpoint of the Palmo Plantar Investigator’s Global Assessment was sustained over 2·5 years with 59·2% (95% CI: 43·5-74·1) and 52·5% (35·1-69·6) of subjects in the secukinumab 300 and 150 mg groups, respectively, achieving clear or almost clear palms and soles (ppIGA 0/1).
At 2·5 years, the mean palmoplantar Psoriasis Area and Severity Index was reduced with both secukinumab 300 mg (-74·7%) and 150 mg (-61·6%).
The safety profile was favourable and similar to previous studies.
Above results were also confirmed in the 2PRECISE study [9] which was a phase 3b multicentre, randomised, double-blind, placebo-controlled, parallel-group study comparing treatment with 300 mg of secukinumab (n = 79), 150 mg of secukinumab (n = 80), and placebo (n = 78) in subjects with moderate-to-severe palmoplantar psoriasis over a period of 52 weeks. Patients with PPP who were treated with secukinumab, 300 mg, showed benefit in terms of Palmo Plantar Area Severity Index75 responses over 52 weeks and improved quality of life.
In this 24-week, double-blind, phase 3b study [10], 102 patients with scalp psoriasis were randomised 1:1 to subcutaneous secukinumab 300 mg or placebo at baseline, weeks 1, 2, and 3, and then every 4 weeks from week 4 to 20. The primary efficacy variable was 90% improvement of Psoriasis Scalp Severity Index (PSSI) score from baseline to week 12.
At week 12, PSSI 90 (secukinumab 300 mg vs placebo, 52.9% vs 2.0%) and Investigator's Global Assessment modified 2011 scalp responses of 0 or 1 (secukinumab 300 mg vs placebo, 56.9% vs 5.9%) were significantly greater with secukinumab 300 mg than placebo (p < 0.001 for both).
The median time to 50% reduction in PSSI score was 3.29 weeks with secukinumab 300 mg. The safety profile of secukinumab was consistent with the previous phase 3 studies.
One of the study limitations was that there was no active comparator arm.
The TRANSFIGURE trial [11] assessed the superiority of secukinumab over placebo in clearing nail psoriasis as assessed by the Nail Psoriasis Severity Index (NAPSI) at week 16 and over time, up to week 132.
In this double-blind, randomised, placebo-controlled study in patients with moderate-to-severe plaque and nail psoriasis, the primary objective of NAPSI was met with both doses of secukinumab which was superior to placebo at week 16 (NAPSI improvements of -45·3%, -37·9% and -10·8% for secukinumab 300 mg and 150 mg and placebo, respectively, p < 0·001).
Significant improvements were seen in patients' quality of life: the NAPPA (Nail Assessment in Psoriasis and Psoriatic Arthritis )-Quality of Life total score median decreases at week 16 were 60·9%, 49·9% and 15·8% for secukinumab 300 mg and 150 mg and placebo, respectively (p < 0·001). Improvement in nail psoriasis continued to week 32. The most common adverse events were nasopharyngitis, headache and upper respiratory tract infections.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children