Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. January 2018.
Introduction Uses How it works How to use Pre-treatment evaluation Use in specific populations Adverse effects Drug interactions Contraindications
Guselkumab is a biological treatment indicated for moderate-to-severe psoriasis.
The US Food and Drug Administration (FDA) has approved guselkumab (Tremfya™, Janssen Biotech, PA, USA) for the treatment of adults living with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Guselkumab is the first and only approved biological therapy that selectively blocks only interleukin 23 (IL-23), a cytokine that plays a key role in plaque psoriasis.
Applications seeking approval of guselkumab in the European Union, Japan, and other countries are currently under review.
In July 2020, guselkumab was approved by the FDA for the treatment of psoriatic arthritis.
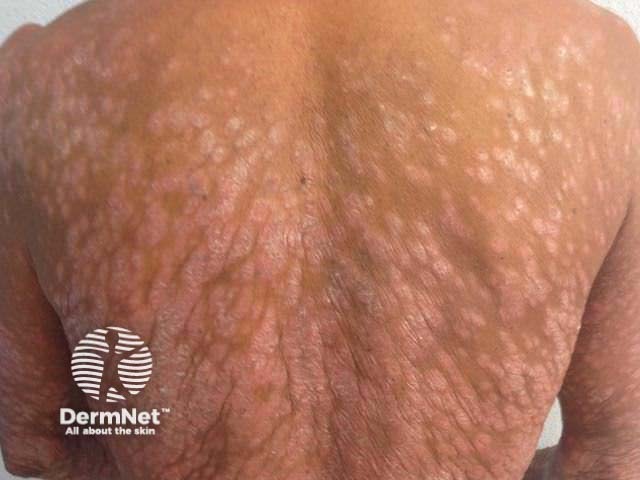
Severe psoriasis affecting back
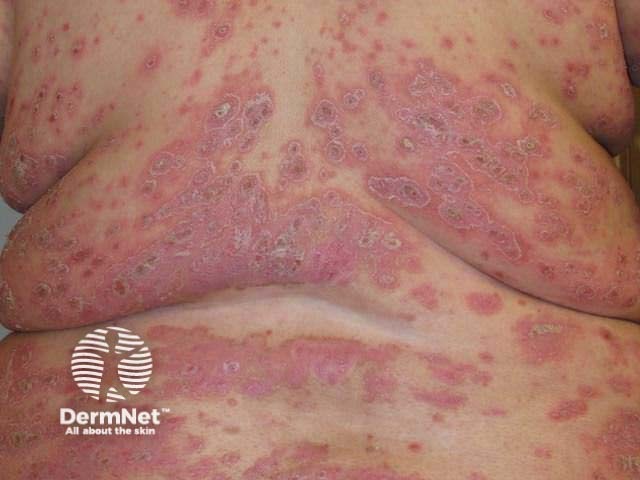
Severe psoriasis
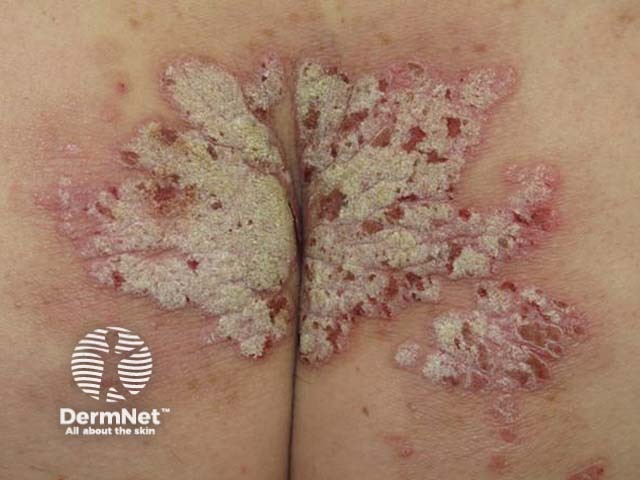
Severe psoriasis buttocks
The safety and effectiveness of guselkumab in paediatric patients < 18 years of age have not been evaluated.
Clinical studies with guselkumab did not include sufficient numbers of patients aged 65 years to determine whether they respond differently from younger subjects.
No trials have been conducted to assess the effect of hepatic or renal impairment on the pharmacokinetics of guselkumab.
The most common adverse reactions (≥ 1% of patients) reported include:
Guselkumab should not be used in patients that:
Guselkumab is promising for plaque psoriasis in Phase III studies.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).