Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Maria McGivern/Gus Mitchell. January 2018.
In July 2017, the US Food and Drug Administration (FDA) approved guselkumab (Tremfya™; Janssen Biotech, PA, USA) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Guselkumab is the first and only approved biological therapy that selectively blocks only interleukin (IL)-23, a cytokine that plays a key role in plaque psoriasis.
Guselkumab received FDA approval based on results from a clinical development programme that included more than 2,000 patients in the Phase III VOYAGE 1, VOYAGE 2, and NAVIGATE studies.
In September 2017, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorisation in the European Union for the use of guselkumab in the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
Guselkumab is currently unavailable in New Zealand (as of January 2018).
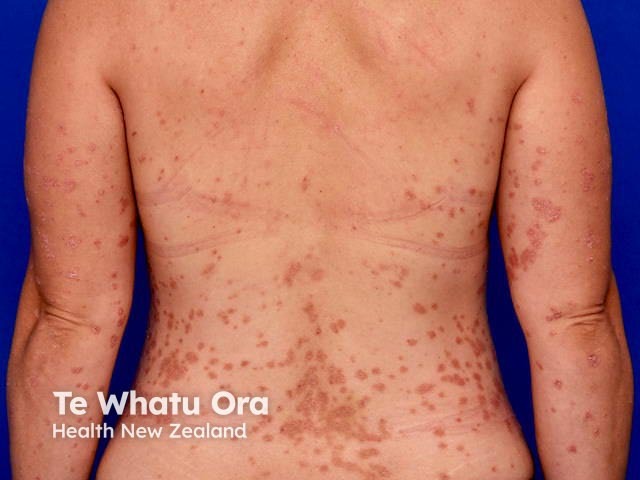
Psoriasis moderate on back
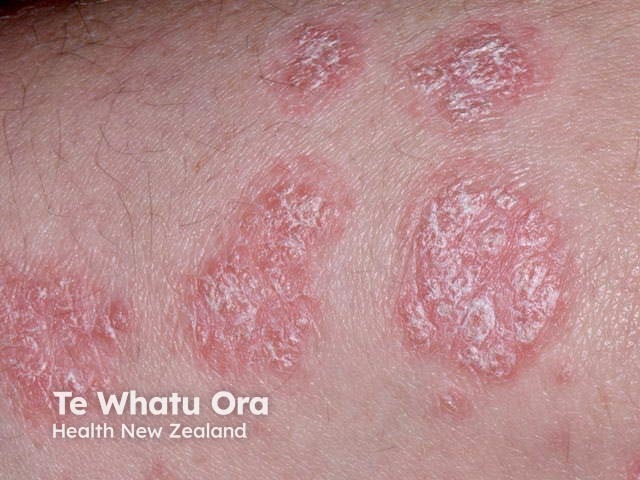
Chronic plaque psoriasis
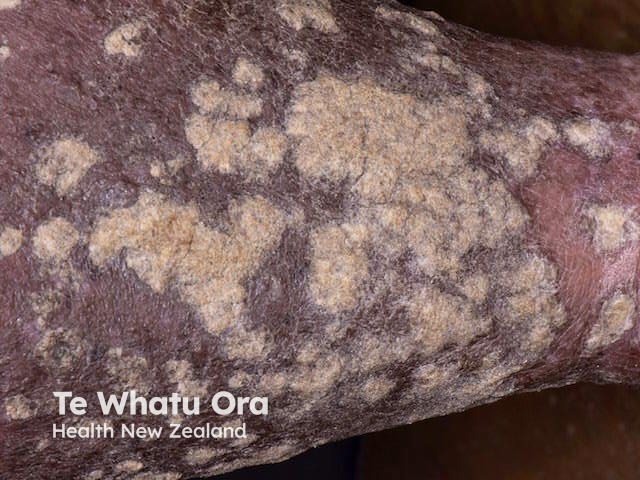
Psoriasis with very thick scale
VOYAGE 1 |
VOYAGE 2 |
|||
Primary endpoint |
Guselkumab |
Placebo |
Guselkumab |
Placebo |
IGA response of 0/1 |
280 (85) |
12 (7) |
417 (84) |
21 (8) |
PASI 90 response |
241 (73) |
5 (3) |
347 (70) |
6 (2) |
IGA, Investigator's Global Assessment score; PASI 90, 90% reduction from baseline in Psoriasis Area and Severity Index composite score.
Table 2 presents the results of an analysis of all the North American sites (US and Canada) in the VOYAGE 1 and 2 trials, demonstrating superiority of guselkumab to adalimumab.
VOYAGE 1 |
VOYAGE 2 |
|||
Endpoint |
Guselkumab |
Adalimumab |
Guselkumab |
Adalimumab |
IGA response of 0 (cleared) or 1 (minimal) |
||||
Week 16 |
97 (84) |
70 (61) |
119 (74) |
50 (62) |
Week 24 |
97 (84) |
62 (54) |
119 (74) |
46 (57) |
Week 48 |
91 (79) |
62 (54) |
NA |
NA |
IGA response of 0 (cleared) |
||||
Week 24 |
61 (53) |
27 (23) |
76 (48) |
23 (28) |
Week 48 |
54 (47) |
28 (24) |
NA |
NA |
PASI 75 response |
||||
Week 16 |
105 (91) |
80 (70) |
132 (83) |
51 (63) |
PASI 90 response |
||||
Week 16 |
84 (73) |
47 (44) |
102 (64) |
34 (42) |
Week 24 |
92 (80) |
51 (41) |
113 (71) |
41 (51) |
Week 48 |
84 (73) |
53 (46) |
NA |
NA |
To evaluate maintenance and durability of response in VOYAGE 2, patients randomised to guselkumab at Week 0 who were PASI 90 responders at Week 28 were re-randomised to guselkumab every 8 weeks or placebo. At Week 48, 89% of patients who continued treatment with guselkumab maintained PASI 90 compared to 37% who were re-randomised to placebo.
Based on the Psoriasis Symptoms and Signs Diary (PSSD) at Week 16, patients treated with guselkumab showed greater improvements in itch, pain, stinging, burning, and skin tightness compared to placebo, in both VOYAGE 1 and 2 trials. A greater proportion of patients treated with guselkumab compared with adalimumab achieved a PSSD symptom score of 0 (symptom-free) at week 24, in both trials.
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials may not reflect the rates observed in practice. Table 3 summarises commonly observed adverse events in the VOYAGE 1 and 2 trials.
Adverse events |
Guselkumab |
Adalimumab |
Placebo |
Upper respiratory infections |
118 (14.3) |
21 (10.7) |
54 (12.8) |
Headache |
38 (4.6) |
2 (1) |
14 (3.3) |
Injection site reactions |
37 (4.5) |
15 (7.7) |
12 (2.8) |
Arthralgia |
22 (2.7) |
4 (2) |
9 (2.1) |
Diarrhoea |
13 (1.6) |
3 (1.5) |
4 (0.9) |
Gastroenteritis |
11 (1.3) |
4 (2) |
4 (0.9) |
Tinea infection |
9 (1.1) |
0 |
0 |
Herpes simplex infection |
9 (1.1) |
0 |
2 (0.5) |
Elevated liver enzymes were reported more frequently in the guselkumab group (2.6%) than in the placebo group (1.9%). Of the 21 patients who reported elevated liver enzymes in the guselkumab group, all events except one were mild-to-moderate in severity and none of the events led to discontinuation of the drug.
The frequency of AEs was similar to the safety profile observed during the first 16 weeks of treatment.
Efficacy parameter |
Guselkumab |
Ustekinumab |
P value |
Mean number of visits at which patients achieved an IGA response of 0 or 1 and at least a two-grade improvement (relative to Week 16) from Week 28 through to Week 40 |
1.5 |
0.7 |
p≤ 0.001 |
Mean number of visits at which patients achieved an IGA response of 0 from Week 28 through to Week 40 |
0.9 |
0.4 |
p ≤ 0.001 |
Mean number of visits at which patients achieved a PASI 90 from Week 28 through to Week 40 |
2.2 |
1.1 |
p ≤ 0.001 |
Number of patients with an IGA response of 0 or 1 and ≥ 2-grade improvement (relative to Week 16) at Week 28 |
42 |
19 |
p ≤ 0.001 |
Number of patients with a PASI 90 response at Week 28 |
65 |
30 |
p ≤ 0.001 |
Number of patients with DLQI score > 1 at Week 16 |
103 |
105 |
p = 0.002 |
Number of patients with DLQI score of 0 or 1 at Week 52 |
40 |
20 |
|
Number of patients with a PSSD score > 0 at Week 16 |
123 |
126 |
|
Number of patients with a PSSD score of 0 at Week 52 |
25 |
12 |
p 0.05 |
DLQI, Dermatology Life Quality Index; Investigator's Global Assessment score; PASI 90, 90% reduction from baseline in Psoriasis Area and Severity Index composite score; PSSD, Psoriasis Symptoms and Signs Diary.
Adverse event |
Guselkumab |
Ustekinumab |
Infections |
57 (41.5%) |
47 (35.3%) |
Nasopharyngitis |
23 (17%) |
23 (17.3%) |
Respiratory tract infections |
15 (11.1%) |
11 (8.3%) |
Injection site reaction |
7 (1.1 %) |
0 |
Cardiovascular event |
2 (1.5 %) |
1 (0.8%) |
Malignancy |
2 (1.5%) |
0 |
Approved datasheets are the official source of information for medicines, including approved uses, doses, and
safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).