Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Reactions Treatments Lesions (cancerous)
Author: Dr Peru Urigoitia-Ugalde, Onco-Dermatology Fellow; Prof Fernandez-Penas, Dermatologist, Westmead Hospital, NSW, Australia. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. January 2020.
Introduction
Introduction - targeted therapies
Introduction - immunotherapy
How they work
Introduction
BRAF inhibitors
MEK inhibitors
Definition
Anti-CTLA4 antibody
Anti-PD1 antibodies
Combination therapy
The introduction of targeted cancer therapies and immunotherapy has revolutionised the management and prognosis of metastatic melanoma, significantly improving drug response rates and disease-free survival.
These drugs are associated with a range of adverse effects, and cutaneous toxicities are the most frequently observed.
Cutaneous
Subcutaneous
Lymphatic
Targeted therapies (BRAF inhibitors and MEK inhibitors) are drugs to treat BRAF-mutant metastatic melanoma. Activating mutations in the BRAF gene are present in 50–70% of melanomas. The most common mutation occurs at position 600, where valine (V) is substituted by glutamic acid (E) (V600E) [1].
Immunotherapy includes anti-cytotoxic T lymphocyte antigen 4 (CTLA4) antibodies and programmed cell death protein 1 (PD1) antibodies. These drugs are used to treat metastatic melanoma, renal cell carcinoma, non-small cell lung cancer, head and neck squamous cell carcinoma, and others.
Mutations in the BRAF gene lead to overactivation of the mitogen-activated protein kinase (MAPK) pathway, which plays a key role as a regulator of cell growth, differentiation, and survival. Inhibition of the BRAF kinase and the mitogen-activated extracellular signal-regulated kinase (MEK) leads to inhibition of cellular proliferation.
The mechanism behind most of the cutaneous toxicities associated with BRAF inhibitors is thought to be a paradoxical activation of the mitogen activated kinase (MAPK) in cells without the BRAF mutation, which leads to cellular hyperproliferation [2].
CTLA4 and PD1 are immune-checkpoint receptors that down-regulate T-cell immune function and promote self-tolerance.
BRAF inhibitors include vemurafenib and dabrafenib. Cutaneous adverse effects are common.
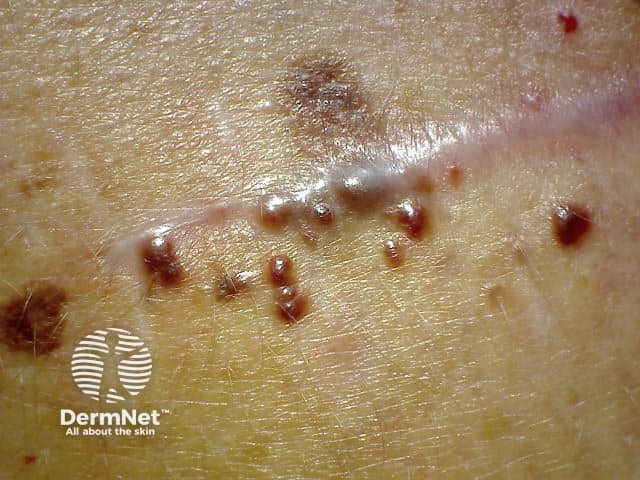
Cutaneous squamous cell carcinoma (SCC) frequently occurs in patients treated with a BRAF inhibitor as single agent. The incidence decreases with the addition of a MEK inhibitor. The majority of the SCC develop during the first 3 months of therapy, in sun-damaged and non-sun-damaged locations. Most SCC are well-differentiated or are a keratoacanthoma type. Excision is the standard treatment with a high rate of cure. Oral acitretin can be used as a preventive agent, as it reduces epidermal proliferation and does not interfere with the action of the targeted therapy.
Verrucal keratosis presents as a hyperkeratotic papule resembling a viral wart or small keratoacanthoma. They are considered premalignant and therefore precursors to cutaneous SCC, and should be monitored. Treatment options include cryotherapy or surgery. Acitretin can be used as a preventive therapy.
Transient acantholytic dermatosis (Grover disease) is a common cutaneous eruption associated with BRAF inhibitors. It presents on the trunk as rapid-onset, itchy papules with a crusted top. Treatment includes topical corticosteroids, keratolytic agents (such as urea and salicylic acid) and acitretin for long-term control.
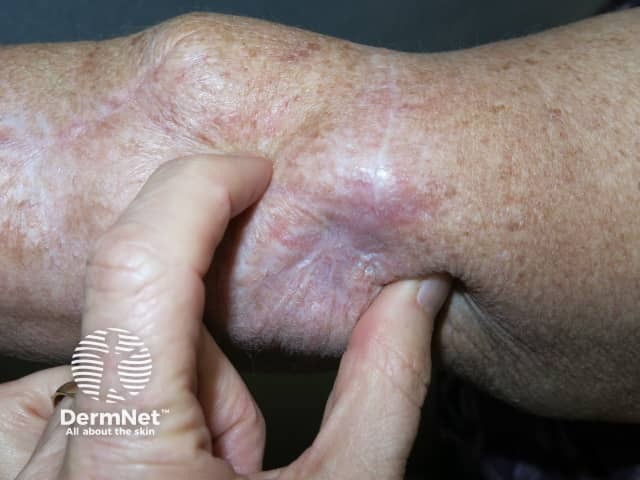
Palmoplantar keratoderma presents as hyperkeratotic plaques or calluses over areas of pressure or friction (mainly on the feet). Blisters are uncommon. The keratoderma can be very painful and limit the patient’s daily activities. Treatment with keratolytic agents (such as urea or salicylic acid) and potent topical corticosteroids are indicated (eg, clobetasol propionate ointment). Patients should be educated to avoid friction and to wear wide, comfortable shoes.
Folliculitis due to vemurafenib
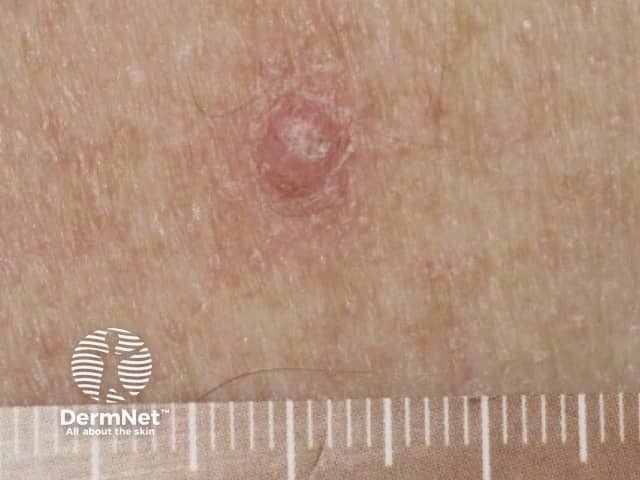
Papilloma due to vemurafenib
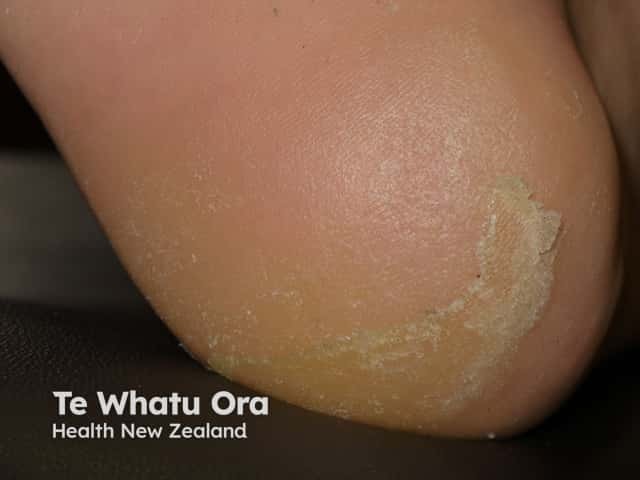
Keratoderma due to vemurafenib
Changes in melanocytic lesions have been reported in patients on BRAF inhibitors, including the development of new naevi and regression or hyperpigmentation of existing naevi. An increased frequency of new primary melanoma has also been reported. These new melanomas have been shown to be wild type for the BRAF mutation, suggesting that paradoxical activation of the MAPK pathway may play a role [4].
Drug-induced pruritus is commonly reported in patients taking BRAF inhibitors. Pruritus can be secondary to drug-induced dry skin or transient acantholytic dermatosis. General measures such as the use of soap-free cleansers and emollients are recommended. Topical corticosteroids can be used if dermatitis develops.
Drug-induced photosensitivity is more commonly seen with vemurafenib than dabrafenib and is induced by exposure to ultraviolet (UV) A radiation. Sun protection, including the use of broad-spectrum high protection factor sunscreen, is recommended.
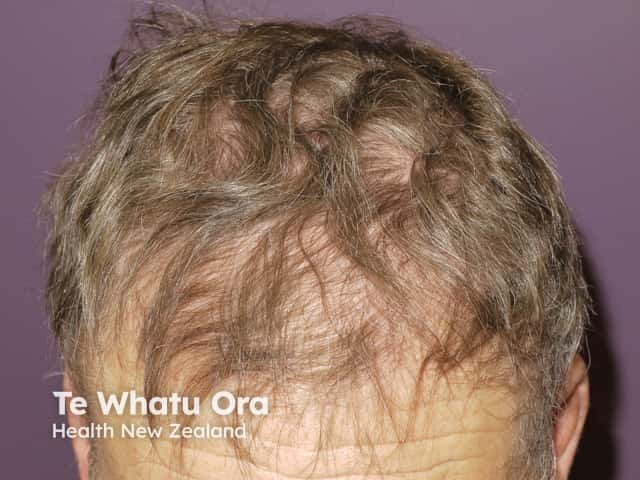
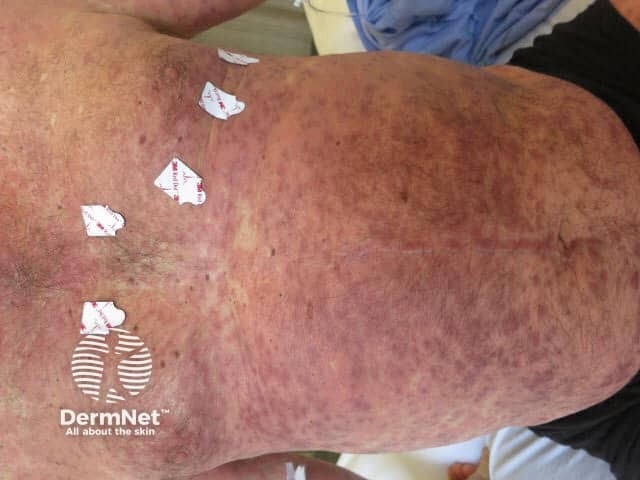
Alopecia due to vemurafenib
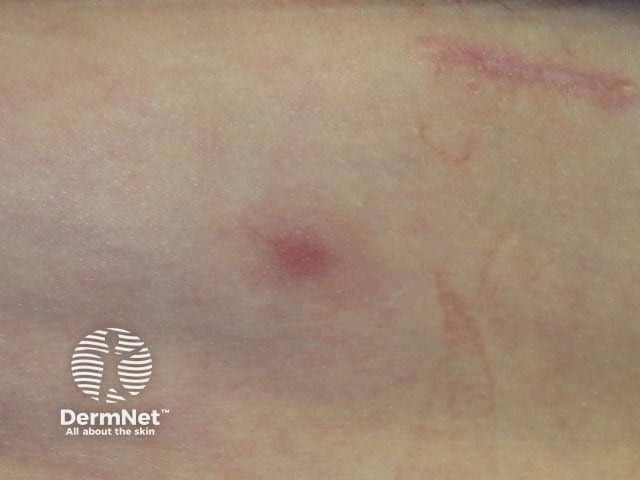
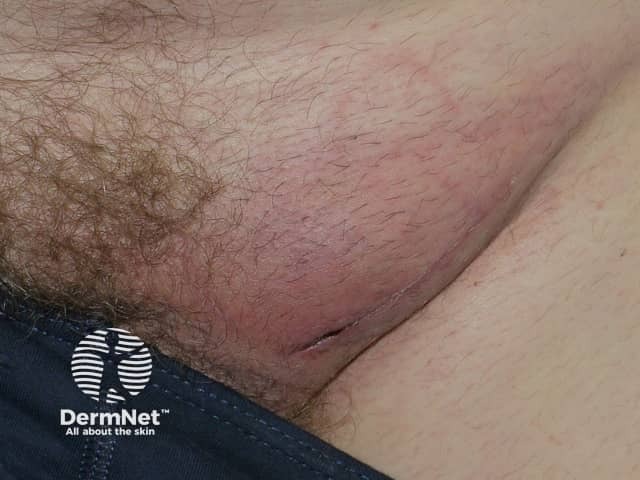
Panniculitis due to vemurafenib
MEK inhibitors include trametinib and cobimetinib. These drugs are usually administered in combination with BRAF inhibitors.
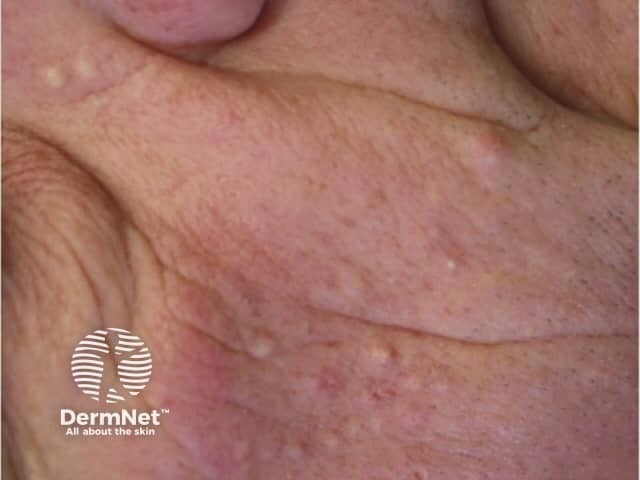
Acneiform drug eruptions are the most common cutaneous adverse events associated with MEK inhibitor monotherapy, appearing in a seborrhoeic distribution (face, scalp, upper chest, and back). Papules and pustules are present, but comedones are absent, unlike acne vulgaris. Oral tetracyclines (doxycycline, minocycline) and isotretinoin are used to prevent and treat acneiform eruptions.
Drug-induced pruritus; topical corticosteroids may be used for symptomatic treatment.
When melanoma started to develop resistance to single-agent BRAF inhibitors, MEK inhibitors were introduced as a combination therapy (dabrafenib + trametinib, vemurafenib + cobimetinib). The addition of a MEK inhibitor to a BRAF inhibitor improves the blockade of the mitogen-activated protein kinase (MAPK) pathway.
Combination therapies increase response rates and show an improved toxicity profile in comparison to monotherapy. There is a noticeable reduction in squamoproliferative lesions (cutaneous SCC, verrucal keratosis, acantholytic dermatosis, palmoplantar keratoderma), hair follicle changes, and acneiform eruptions [4,5].
Folliculitis is the most common adverse effect of BRAF inhibitor and MEK inhibitor combination therapy and is usually mild. An antiseptic wash is usually sufficient to treat it [4].
Ipilimumab is an anti-CTLA4 antibody.
Ipilimumab commonly causes a maculopapular exanthem or morbilliform drug eruption. It is usually mild to moderate in severity, appearing on the trunk and extremities. Mild cases can be managed without discontinuation or dose reduction of ipilimumab. Topical corticosteroids or topical calcineurin inhibitors can be used. In more severe cases, ipilimumab should be discontinued and oral prednisone may be indicated.
Drug-induced vitiligo has been reported with ipilimumab. Patients should be educated about sun protection. An association between the development of vitiligo and better disease prognosis has been reported in multiple studies [5].
Other cutaneous adverse effects of immunotherapy can include:
Anti-PD1 antibodies include nivolumab and pembrolizumab.
They are better tolerated than anti-CTLA4 antibodies. However, anti-PD1 antibodies show similar immune-related adverse reactions [4,6]. Skin reactions can include:
See also Cutaneous adverse effects of checkpoint inhibitors.
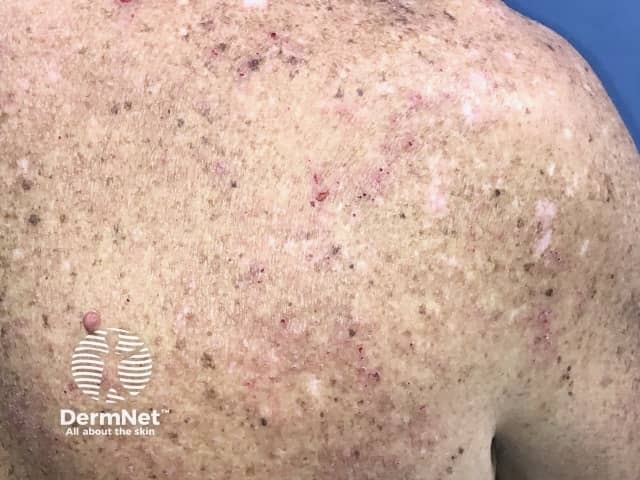
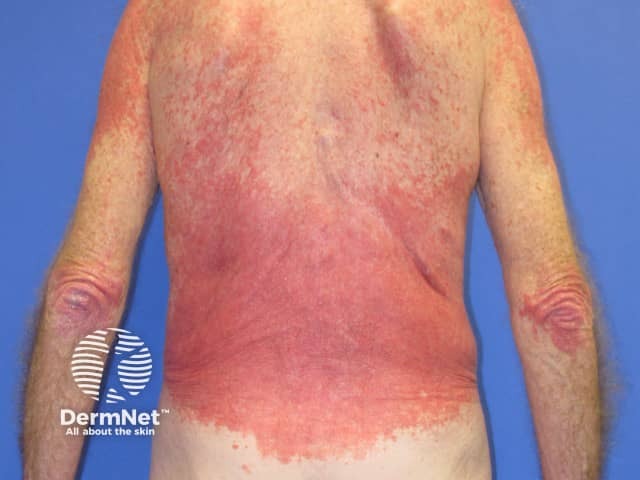
Eczema induced by pembrolizumab
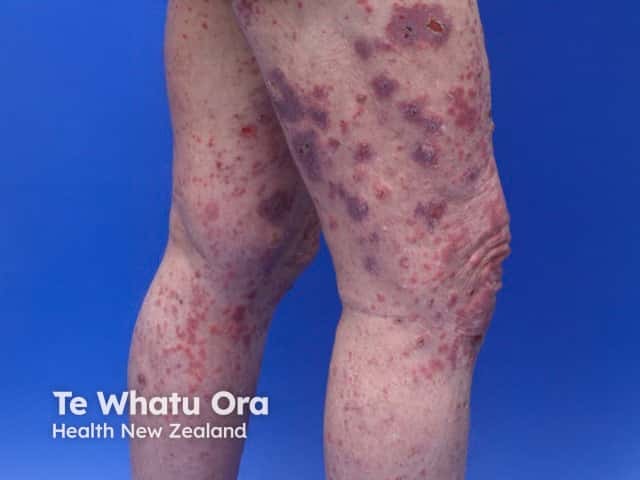
Bullous pemphigoid induced by pembrolizumab
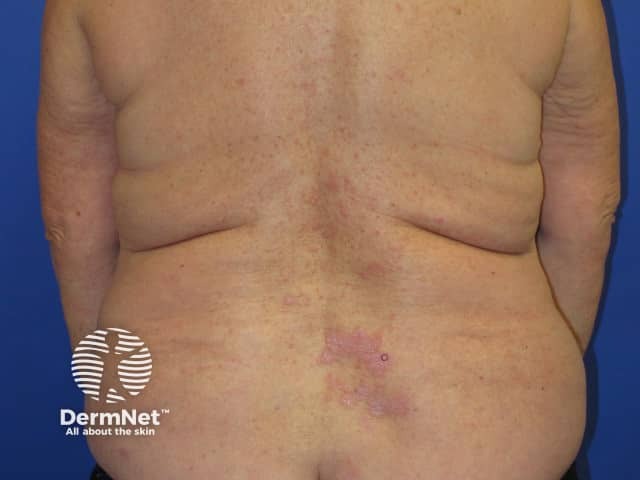
Nivolumab-induced lichenoid dermatitis
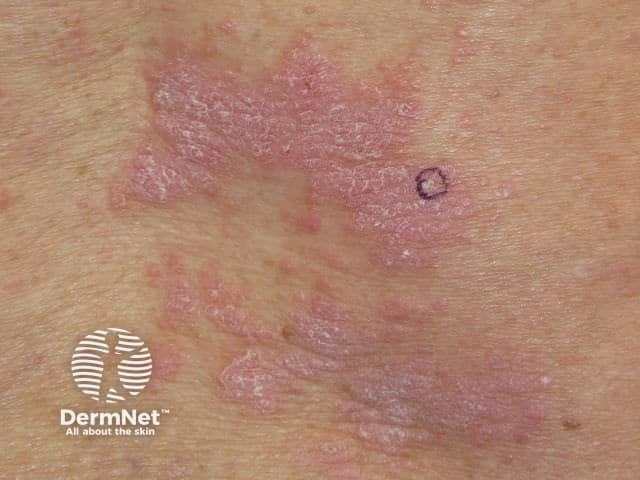
Nivolumab-induced lichenoid dermatitis
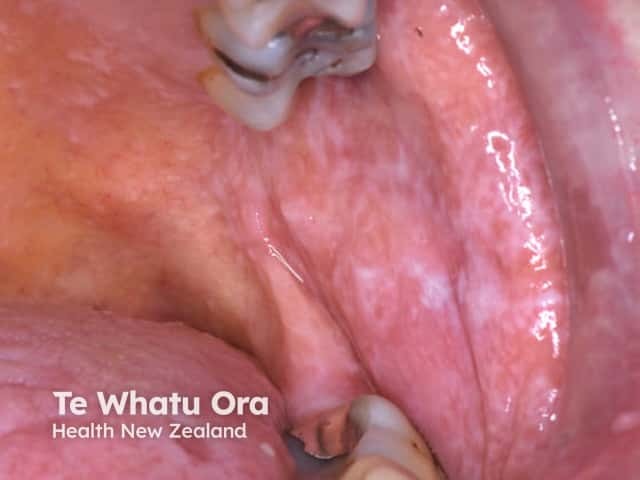
The combination of anti-CTLA4 and anti-PD1 antibodies shows superior efficacy compared to monotherapy, but it is also associated with an increase in adverse events [7]. The most common is drug-induced pruritus. Others include morbilliform eruption and lichenoid drug eruption. Severe cutaneous adverse reactions such as acute generalised exanthematous pustulosis (AGEP) have been reported.