Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Jacqueline Deen, Dermatology Principal House Officer, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. November 2019.
Introduction Uses - topical calcineurin inhibitors Uses - oral calcineurin inhibitors Cutaneous side effects - topical calcineurin inhibitors Cutaneous side effects - oral calcineurin inhibitors
Calcineurin inhibitors are drugs that work by inhibiting the calcium-dependent protein phosphatase calcineurin, an enzyme that results in activation of T lymphocytes through the upregulation of interleukin-2 and related cytokines, and leads to immunosuppression [1].
The calcineurin inhibitors tacrolimus and pimecrolimus are indicated for the treatment of atopic dermatitis.
They are commonly used off-label in dermatology for:
Topical calcineurin inhibitors are free of many adverse effects of topical corticosteroids, such as striae, cutaneous atrophy, glaucoma, rebound flares and systemic absorption, or the potential for hypothalamic pituitary–adrenal axis (HPA) suppression.
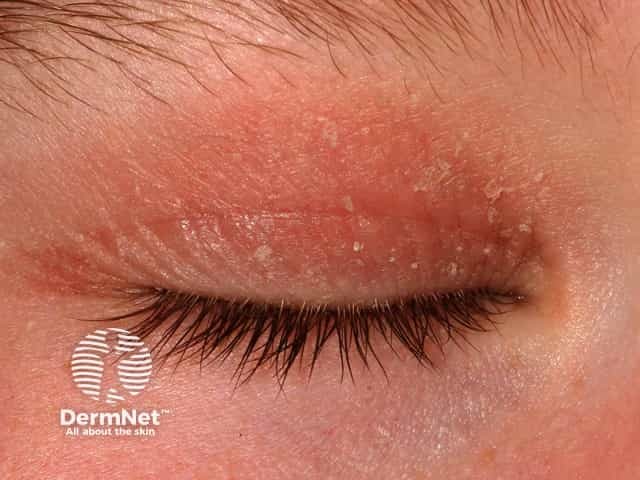
Atopic dermatitis of the eyelid
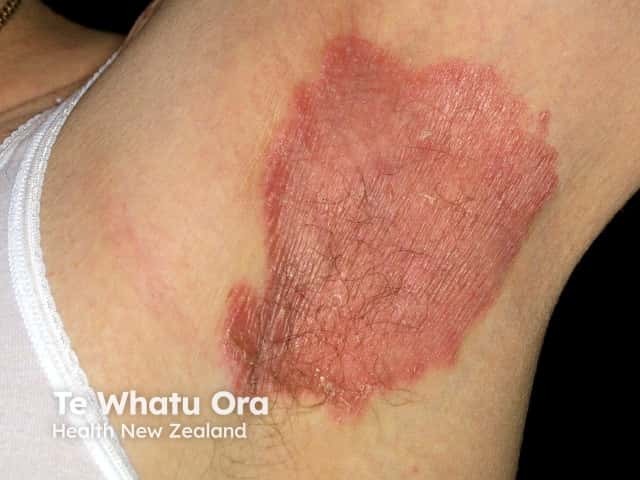
Flexural psoriasis
Oral calcineurin inhibitors are commonly used for immunosuppression after solid organ transplantation. Occasionally, ciclosporin and tacrolimus may also be used to treat immune-mediated diseases including psoriasis and atopic dermatitis; however, their use is mainly restricted to patients who have failed to respond to conventional therapy [3].
The most commonly reported side effect of topical calcineurin inhibitors is local skin irritation (burning, pruritus, and erythema) at the application site. However, this is usually transient and decreases over time (usually within one month).
Other adverse effects may include:
There is no evidence to support the theoretical concern that topical calcineurin inhibitors may increase the risk of malignancy, such as basal cell carcinoma, cutaneous squamous cell carcinoma, melanoma and cutaneous lymphoma. Information reporting this risk is based on data from animal studies, case reports in a small number of patients, and knowledge of how these drugs work [2,4,5].
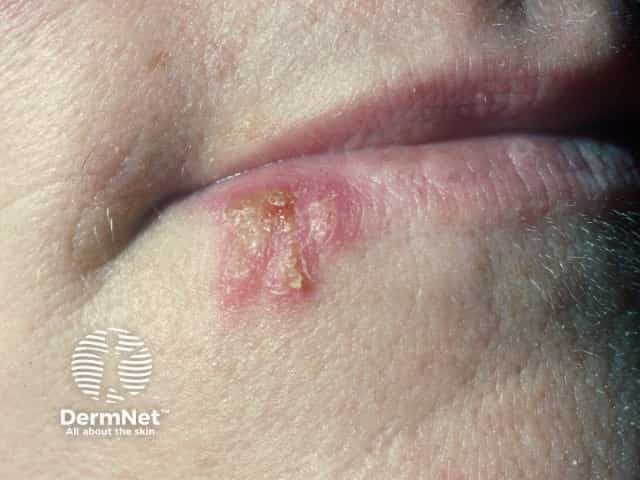
Herpes simplex labialis
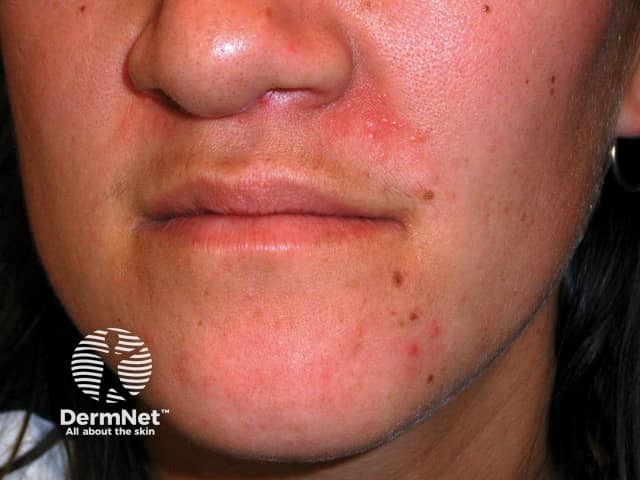
Perioral dermatitis
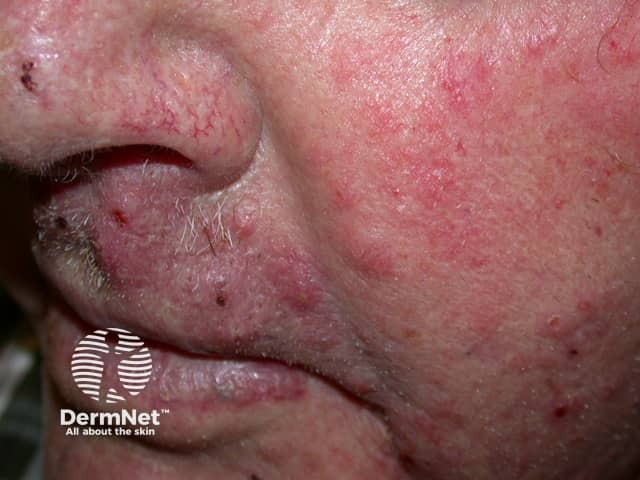
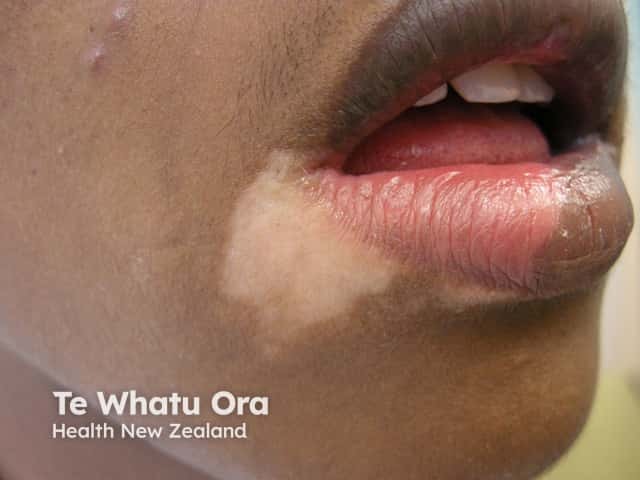
Tinea incognita - face
The adverse effects of tacrolimus and ciclosporin are generally similar. These include:
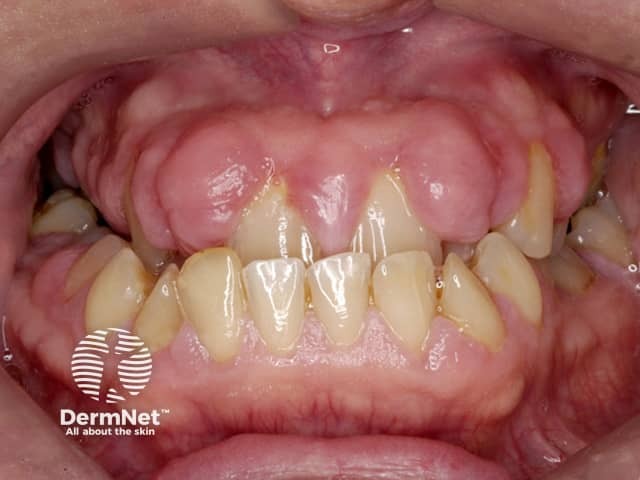
Gum hypertrophy due to ciclosporin
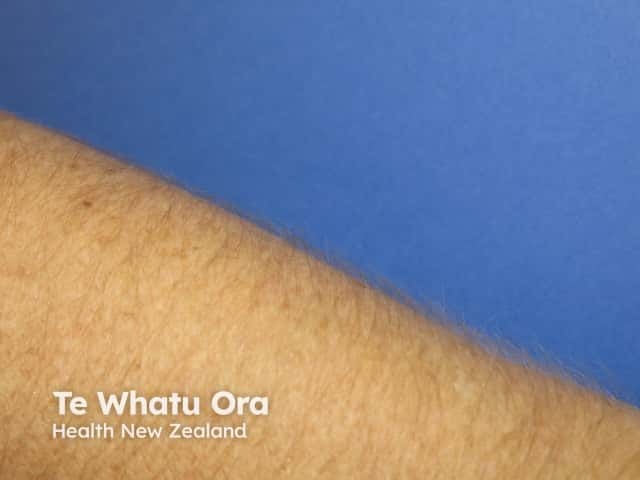
Hypertrichosis
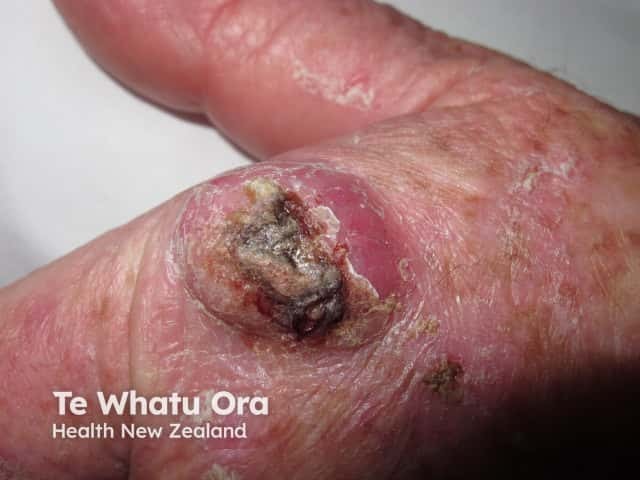
Cutaneous squamous cell carcinoma