Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Delwyn Dyall-Smith FACD, Dermatologist, 2009.
Introduction
Demographics
Clinical features
Diagnosis
Treatment
Proposed mechanisms
Alopecia from drugs is a usually reversible nonscarring diffuse hair loss that occurs within days to weeks of starting a new medication or changing the dose.
The development of hair loss and severity depend both on the drug and individual predisposition. Some drugs cause hair loss in most patients receiving an appropriate dose. Other drugs are only occasionally responsible for hair loss.
There are two types of drug-induced hair loss:
Anagen effluvium is usually due to chemotherapy drugs and rarely with gold, colchicine or poisoning with arsenic, bismuth, thallium or boric acid.
Telogen effluvium is the mechanism of virtually all other medication-induced hair loss. The list of possible drug causes is very long and includes:
Telogen effluvium may also occur as a result of a serious drug eruption such as Stevens-Johnson syndrome / toxic epidermal necrolysis or drug hypersensitivity syndrome, in which case hair falls out a few weeks to months after the acute illness and slowly regrows again.
Hair loss due to medications is usually diffuse and nonscarring. The hair loss may be ‘patterned’ as seen in male-pattern or androgenetic alopecia or female-pattern alopecia. The scalp is the most common site affected, but all body hair including eyebrows and eyelashes may be lost with chemotherapy.
Anagen effluvium hair loss may become obvious within days to weeks of starting chemotherapy, whereas with telogen effluvium the hair loss usually becomes evident after 2–4 months.
In a study of women having chemotherapy for breast cancer, the average time between starting chemotherapy and hair loss was 4–5 weeks but occurred in some as early as two weeks. The hair loss was maximal in the second cycle with more than 1000 hairs/day being lost in severe cases. Even with chemotherapy, the degree of hair loss can vary between no noticeable effect through to severe rapid, extensive loss, even on the same drugs and regimes.
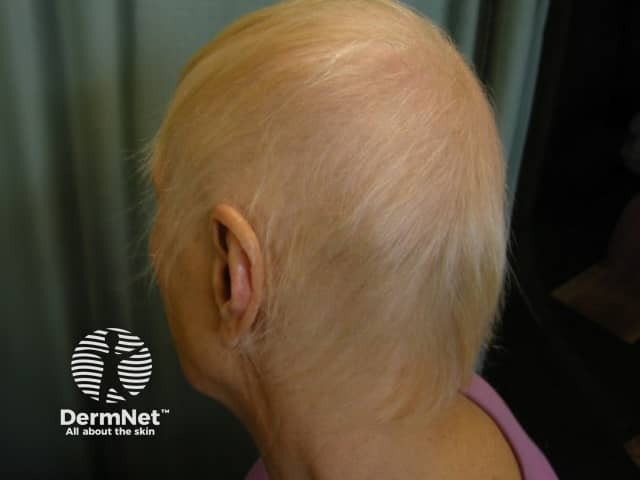
Anagen loss during chemotherapy
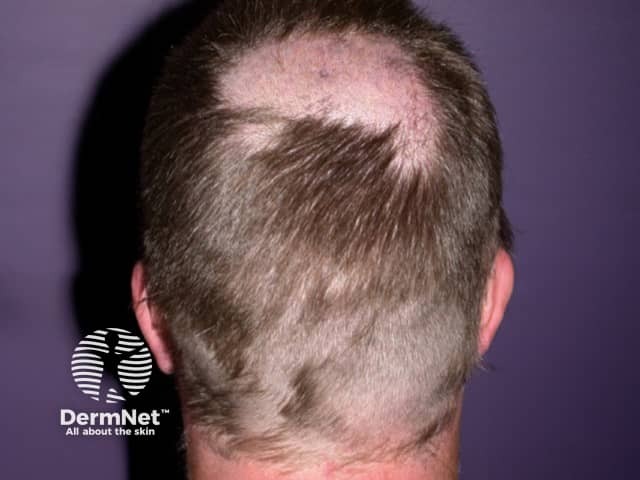
Regrowth following patchy loss associated with itraconazole
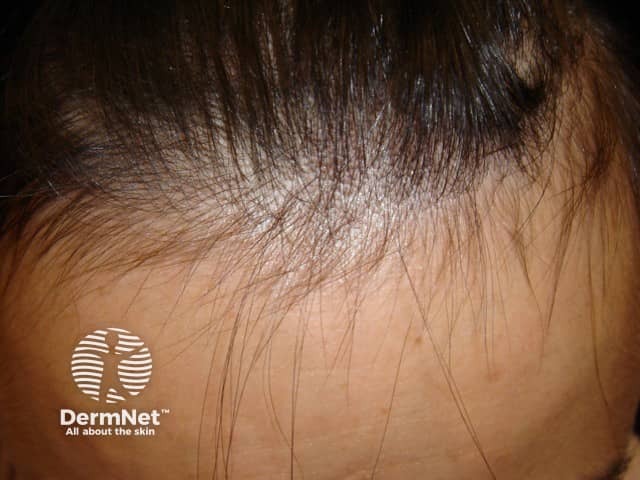
Telogen effluvium regrowth is best seen at the hairline
The only way to confirm suspected drug-induced hair loss is to cease the possible drug for at least three months and observe regrowth. However, the diagnostic steps below may be necessary.
A detailed drug history should be taken in all patients presenting with diffuse nonscarring hair loss, concentrating on the three months before the hair loss was noted. This should include all new medications, any changes in dosages, and also over-the-counter supplements. This list must include chemotherapy, the oral contraceptive pill and hormone replacement therapy. Other causes of diffuse hair loss should also be asked about, such as general health, recent illness or surgery and dietary history. The family history for patterned (androgenetic) hair loss should be noted.
Examination of the scalp should assess the degree and pattern of hair loss, the presence of redness or scaling of the scalp and the length, diameter and breakage of hair shafts.
The hair pull test involves gentle pulling of a cluster of hairs from the base to the tip. Normally only 1-2 hairs come out. In hair shedding conditions, 10-15 hairs may pull out. The pulled hairs can be examined under the microscope for anagen or telogen bulbs, fractures and tapering.
A skin biopsy from an area where the hair is thin may be required to exclude other causes of diffuse hair loss.
If the explanation for hair loss is obscure, it is usual to perform at least blood count, iron studies and thyroid function tests.
Usually, the only treatment required for drug-induced hair loss is to cease the causative drug if it is possible to do so. Once that drug has been ceased, hair shedding settles, although this may take up to 6 months. Evidence of hair regrowth is usually seen within 3–6 months but can take 12–18 months to recover cosmetically.
The most effective treatment to reduce hair loss from chemotherapy to date has been cooling the patient’s head to reduce the blood flow around hair follicles. Hair cooling devices are worn for 30 minutes before infusion of the causative drug, during the infusion, and for 90 minutes afterwards.
Topical calcipotriol was not effective in a trial of breast cancer patients undergoing chemotherapy. In mice, topical minoxidil and topical ciclosporin stimulated regrowth after cyclophosphamide.