Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author(s): Dr Tara Sholji, NHS Lothian, Scotland (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
Mechanism
Application
Contraindications
Pharmacokinetics
Benefits
Disadvantages
Side effects and risks
Berdazimer is a first-in-class nitric oxide (NO)-releasing topical treatment for molluscum contagiosum and under evaluation for use in acne.
Berdazimer 10.3% gel was approved for use by the food and drug administration (FDA) for the treatment of molluscum contagiosum in 2024.
Berdazimer 3.4% gel is currently in Phase III trials for the treatment of acne.
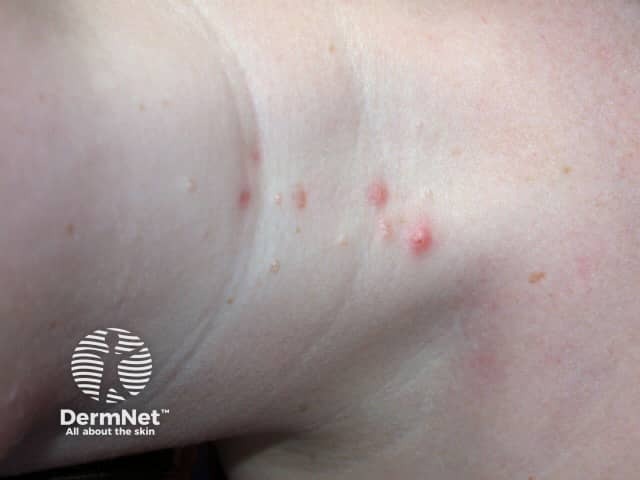
Molluscum contagiosum
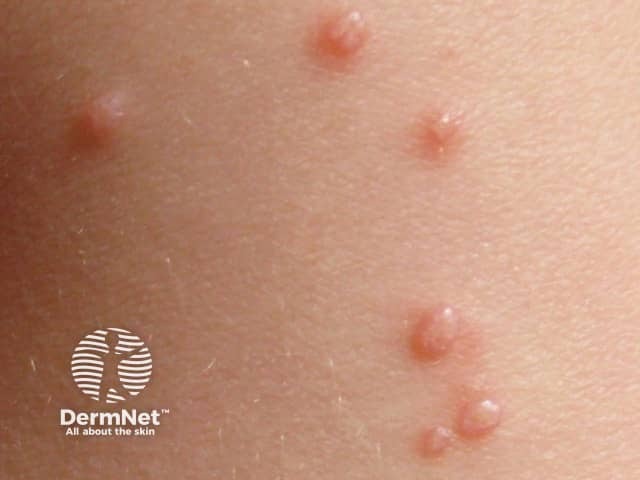
Molluscum contagiosum
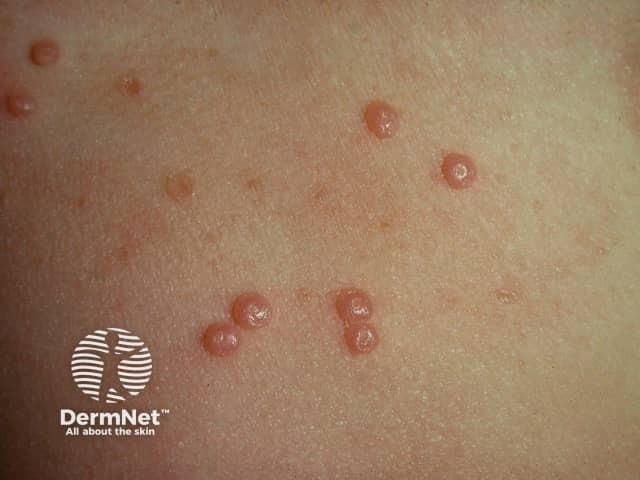
Molluscum contagiosum
Nitric oxide (NO) is naturally produced by the human body and has multiple functions including immune defence. It acts as an antimicrobial via protein nitrosylation that affects immunomodulation, apoptosis, cytokine production, and inflammation. NO can also affect viral replication through cytotoxic reactive oxygen and nitrogen molecules.
Berdazimer consists of two components that act to promote nitric oxide (NO) release:
During trials for molluscum contagiosum, a thin layer of berdazimer 10.3% gel was applied once daily to the lesions.
Hypersensitivity to berdazimer or any of its constituents; otherwise no specific known contraindications have yet been identified.
Measurement of NO metabolite hMAP3 during the Phase I study showed that once-daily application of berdazimer resulted in minimal systemic absorption in children aged 2–12 years old.
The short half-life of unstable gas nitric oxide (NO) and its immunomodulating effects have previously been difficult to use therapeutically, due to challenges with storage and targeted release to treatment sites.
Berdazimer, a first-in-class gel, allows self-application along with the stable release of NO to the affected skin at the time and site of application, minimising systemic exposure.
This is significant as a potential alternative to current molluscum contagiosum treatments including in-clinic curettage, cryotherapy, laser ablation, or chemical removal of the lesions.
In B-SIMPLE4 (a Phase III multi-centre, double-blind, vehicle-controlled, randomised controlled trial), 891 patients aged ≥6 months with 3–70 molluscum contagiosum lesions were randomly assigned to the berdazimer gel or vehicle group for 12 weeks.
In the B-SIMPLE4 trial, berdazimer was generally well tolerated.
Application site reactions (eg, pain, erythema, itching, burning, or stinging) were the most common side effects and mostly mild to moderate; they were the main reason for treatment discontinuation. Overall, these reactions peaked around week 2 of treatment and decreased over the remainder of the study.
No systemic adverse events were observed.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).