Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera B.V.Sc; PhD (Clinical Biochemistry, University of Liverpool, UK), 2012. Updated by Dr Amanda Oakley, 13 March 2014.
Introduction How to use How it works Potential drug interactions Results Future considerations
The Medicines and Healthcare products Regulatory Agency, UK [1] and the European Medicines Agency (EMA) [2] have announced that marketing of Picato® Gel containing ingenol mebutate has been suspended. They have recommended that patients stop using the product pending an investigation into its safety with respect to skin cancer.
See more:
Ingenol mebutate (also called ingenol-3-angelate) is an extract of a common plant, petty spurge or milkweed (Euphorbia peplus). Ingenol mebutate is derived from a cultivar of Euphorbia peplus that is specifically grown in Queensland for this purpose.
It is useful in the treatment of actinic keratoses (actinic keratoses), which are rough spots caused by long term sun exposure.
In January 2012, the US Food and Drug Administration (FDA) approved ingenol mebutate gel for the treatment for actinic keratoses on the face, scalp, trunk and extremities. Ingenol mebutate gel is available in concentrations of 0.015% and 0.05% and is manufactured by LEO Pharma with the trade name Picato®. Ingenol mebutate gel was registered as a prescription medicine by MedSafe for use in New Zealand in October 2013.
The two or three-day course of ingenol mebutate gel compares favourably to several weeks or months needed for other topical therapies used for actinic keratoses, such as 5-fluorouracil cream and imiquimod cream. Treatment can be repeated at a later date if required.

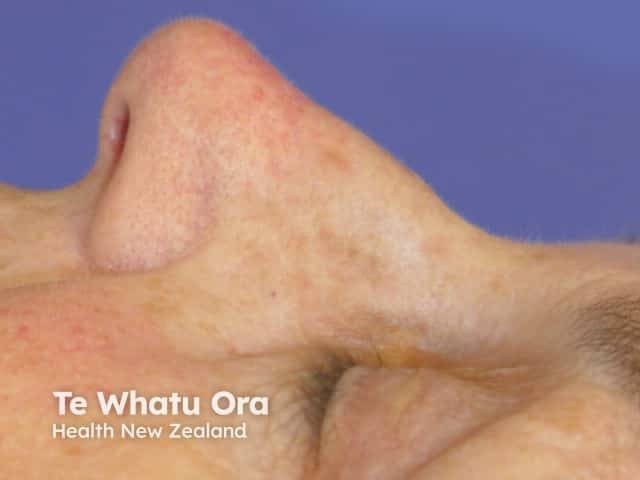
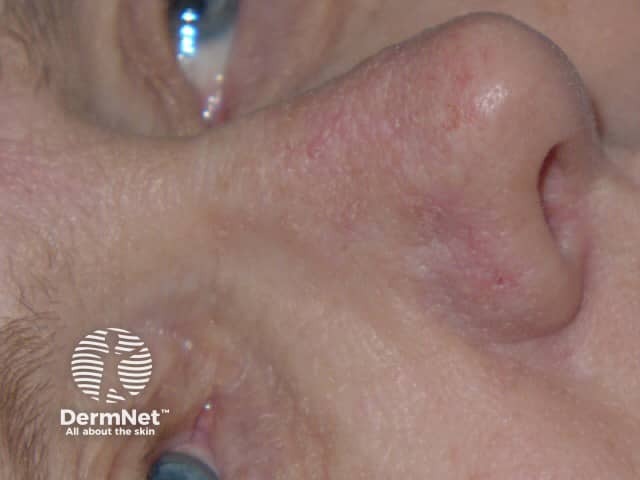
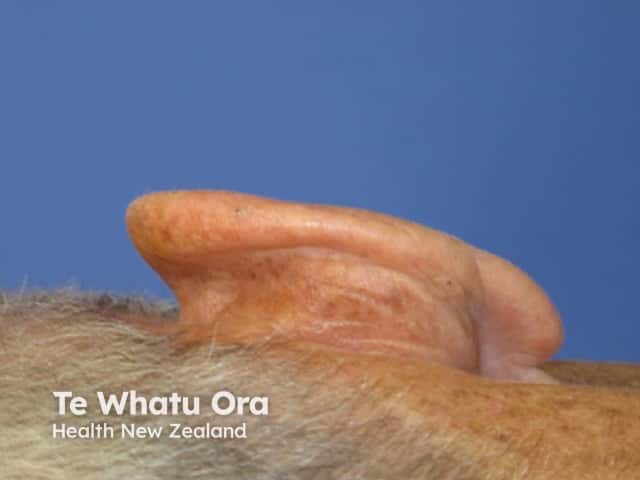
Effects of ingenol mebutate gel on facial actinic keratoses
Effects of ingenol mebutate gel on facial actinic keratoses
Administration of ingenol mebutate gel is not recommended until the skin is healed from any previous drug or surgical treatment. It can be applied at any time of year.
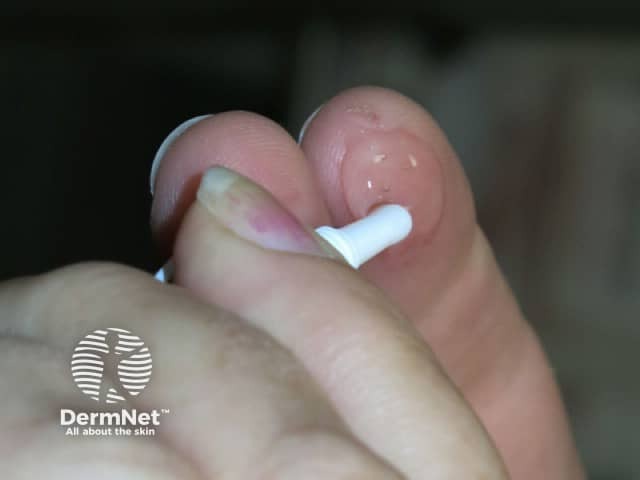
Ingenol mebutate gel is applied to a sun-damaged area. The contents of a single-dose tube will cover about 5 cm x 5 cm of skin. The treatment varies according to the site of the keratoses.
The treated area is allowed to dry for 15 minutes after application and should not be washed or touched for 6 hours after treatment. The treated area can be gently washed after that. Activities that cause excessive sweating should be avoided.
Treated areas become inflamed, often crusted, and then heal over a few days. If blistering or ulceration occurs, the gel should not be reapplied to this site until the skin has fully healed. Moisturiser can be applied as required when the skin peels off.
Day 0 pretreatment |
 Day 0 |
 Day 0 |
 Day 0 |
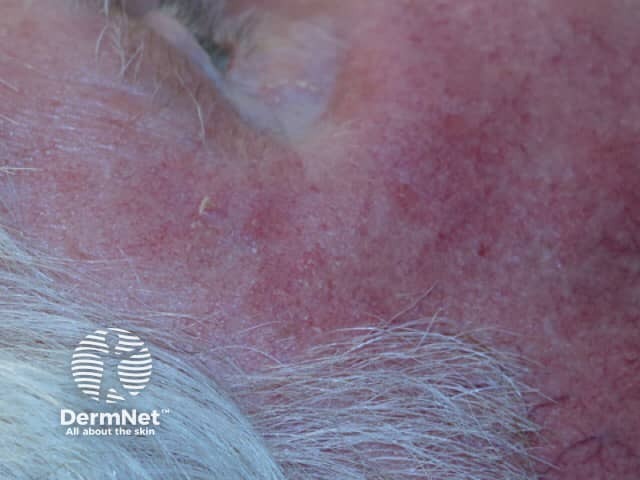 Day 0 |
The worst day |
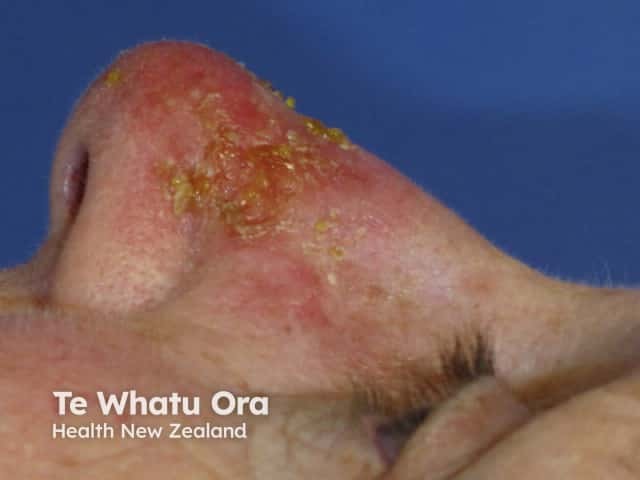 Day 2 |
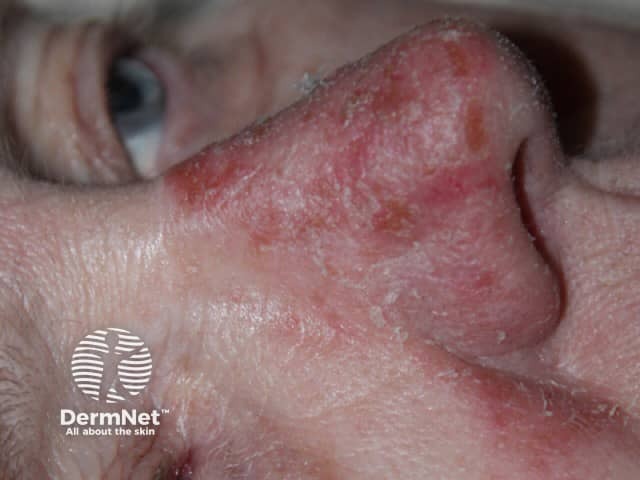 Day 5 |
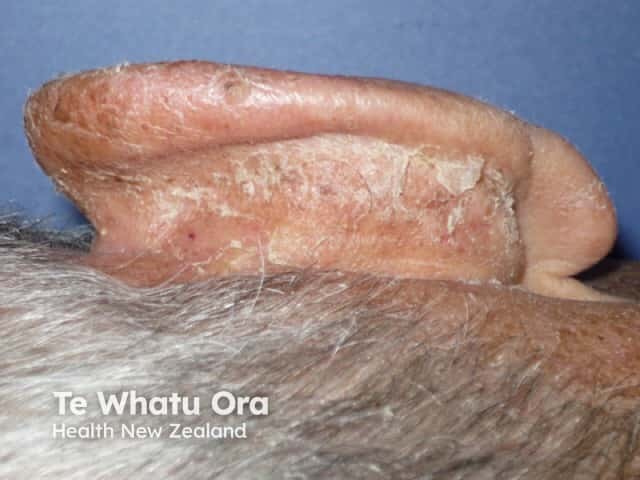 Day 4 |
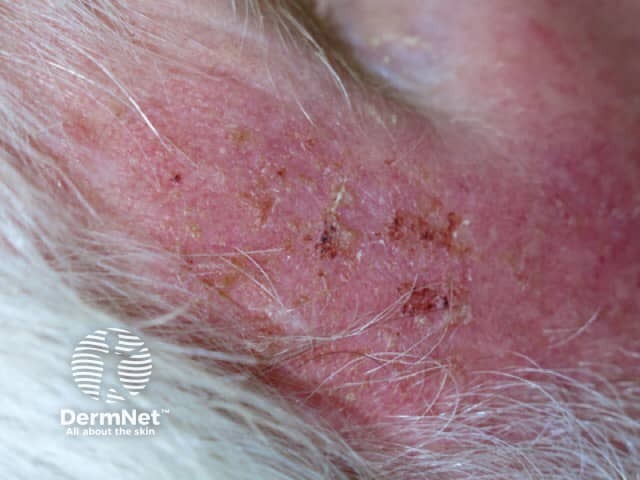 Day 3 |
Follow-up |
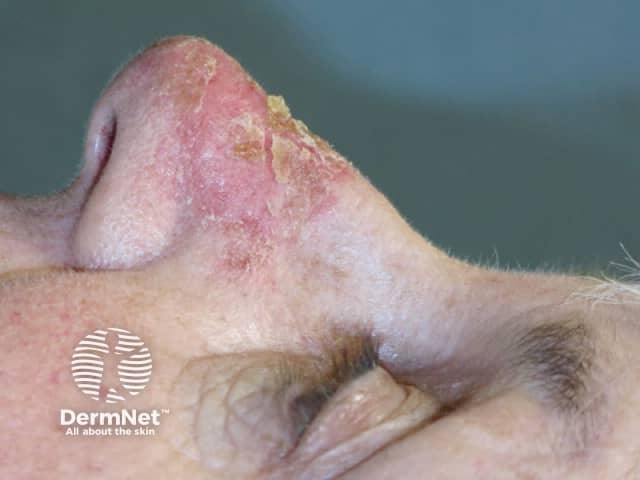 Day 6 |
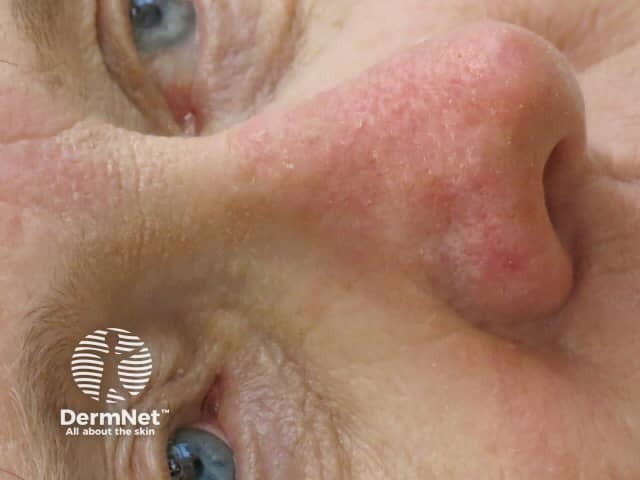 Day 13 |
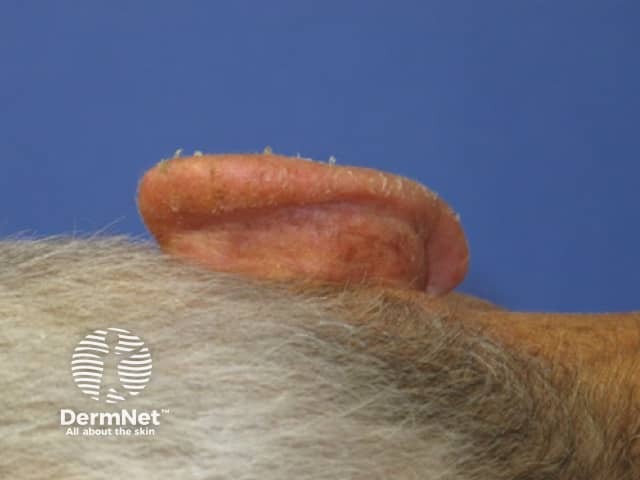 Day 7 |
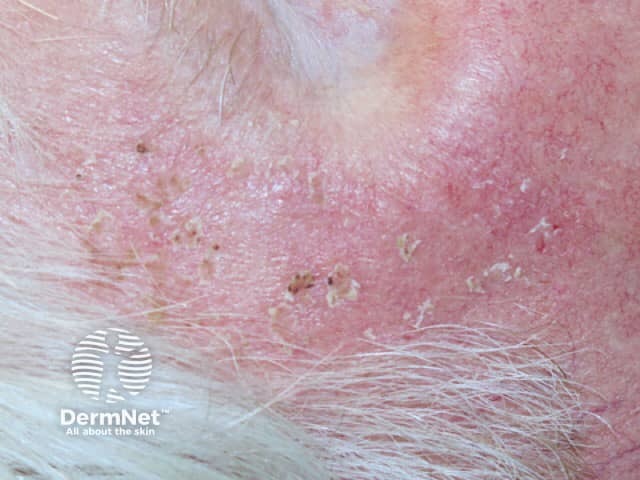 Day 7 |
How ingenol mebutate works on actinic keratoses are incompletely understood. It appears to have a dual mechanism of action:
Link to key clinical-trial evidence
Studies have shown that drug interactions are not likely to be of clinical importance.
Ingenol mebutate gel commonly causes skin reactions at the site of application, such as:
Anaphylaxis and severe contact allergic dermatitis have rarely been reported. A few patients have complained of upper respiratory symptoms and headache.
The periocular area is unsuitable for ingenol mebutate gel because severe eye pain, swelling and drooping of the eyelid can occur if the gel contacts these sites. If accidental exposure occurs, flush eyes with water and seek medical care.
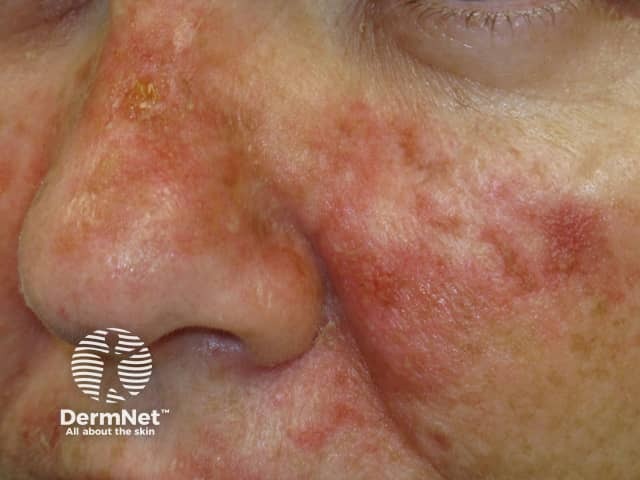
Day 3
There are no adequate and well-controlled studies of ingenol mebutate gel in pregnant women. Ingenol mebutate gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The safety and effectiveness of ingenol mebutate gel have not been established in patients less than 18 years of age, but actinic keratoses are not generally seen in children.
Topical ingenol mebutate is currently in phase II clinical trials for eradicating basal cell carcinoma and squamous cell carcinoma (SCC) in situ (also called intraepidermal SCC or Bowen disease).
A case report described the resolution of a keloid following treatment with 0.05% ingenol mebutate gel daily for two days.
Two of four patients treated for actinic cheilitis had clearance.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children