Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera B.V.Sc; PhD (Clinical Biochemistry, University of Liverpool, UK). Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, January 2017.
Introduction
Evidence of efficacy from trials
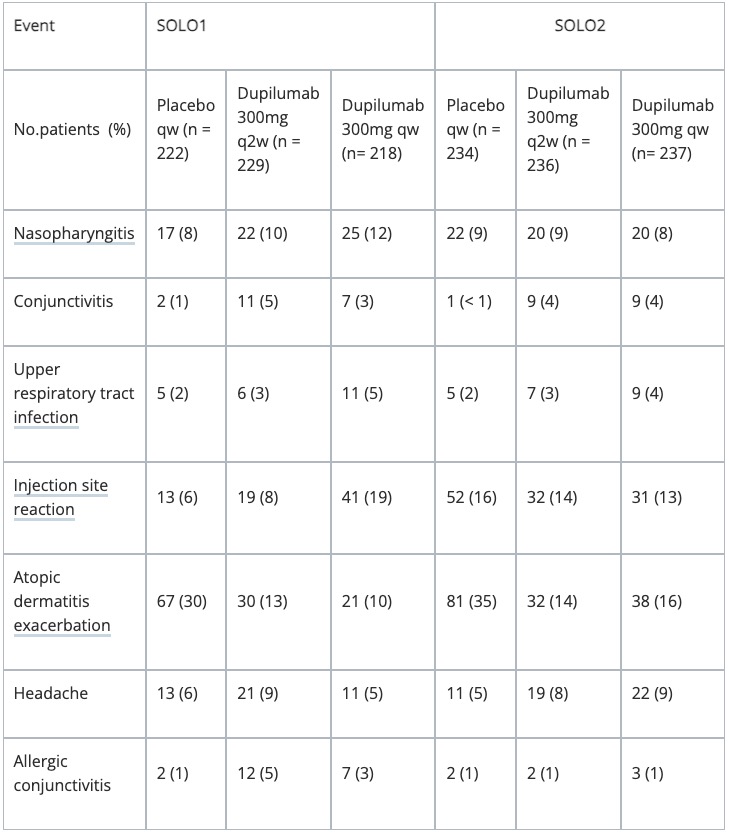
Adverse reactions
Next steps
There have been three pivotal phase 3 clinical studies evaluating dupilumab in the treatment of atopic dermatitis.
Dupilumab (Dupixent™; Sanofi, Paris, France; Regeneron, New York, USA) has shown significant efficacy and a favourable safety profile in two pivotal Phase 3 studies in monotherapy for moderate-to-severe atopic dermatitis, and in concomitant administration with topical corticosteroids.
The US Food and Drug Administration (FDA) has granted dupilumab a breakthrough therapy designation; The Biologics License Application (BLA) for dupilumab was recently accepted for Priority Review by the FDA with a target action date of March 29, 2017.
Dupilumab inhibits signalling of interleukin (IL)-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in atopic dermatitis.
Table 1. Improvements in atopic dermatitis at 16 Weeks
Placebo |
Dupilumab every 2 weeks |
Dupilumab weekly |
P value vs placebo |
|
IGA (SOLO 1) |
10% |
38% |
37% |
0.0001 |
IGA (SOLO 2) |
9% |
36% |
36% |
0.0001 |
EASI-75 (SOLO 1) |
15% |
51% |
53% |
0.0001 |
EASI-75 (SOLO 2) |
12% |
44% |
48% |
0.0001 |
Long-term follow-up – clinical trial evidence from CHRONOS
Co- primary endpoint results at week 16 were:
Co-primary end-point results at week 52 were:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).