Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer. Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, January 2017. Minor update 2024.
Introduction
Uses
How it works
Dosage and administration
Adverse effects
Future
Dupilumab is an innovative first-in-class biological treatment for atopic dermatitis (eczema).
Dupilumab (Dupixent®; Sanofi, Paris, France; Regeneron, New York, USA) is a fully human monoclonal antibody, which has shown significant efficacy and a favourable safety profile in moderate-to-severe atopic dermatitis alone and in combination with topical corticosteroids.
The U.S. Food and Drug Administration (FDA) approved Dupixent® as a treatment for moderate-to-severe atopic dermatitis in adults in March 2017, in patients aged 12–17 in March 2019, and in children age 6–11 in May 2020. It was approved by Therapeutic Goods Administration (TGA) in Australia in January 2018, and it was classified as a prescription medicine by Medsafe in New Zealand in March 2018.
The Biologics License Application (BLA) for dupilumab contains data from three pivotal phase 3 clinical studies evaluating dupilumab as monotherapy and in concomitant administration with topical corticosteroids.
In 2025, dupilumab was approved by the FDA for treatment in adults and adolescents ≥12 years old with chronic spontaneous urticaria (CSU) who remained symptomatic despite H1 antihistamine treatment.
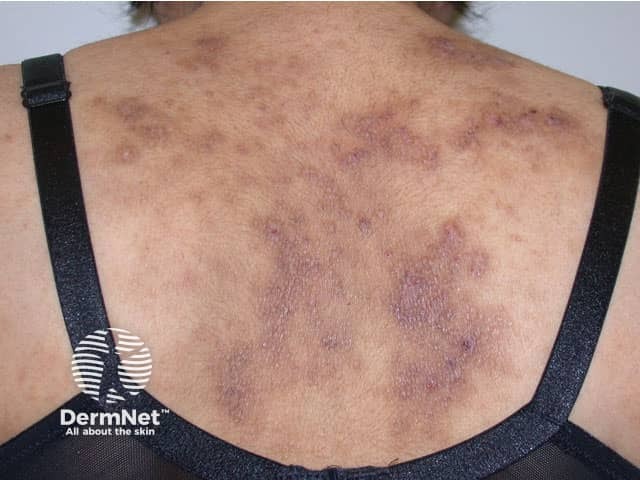
Atopic eczema
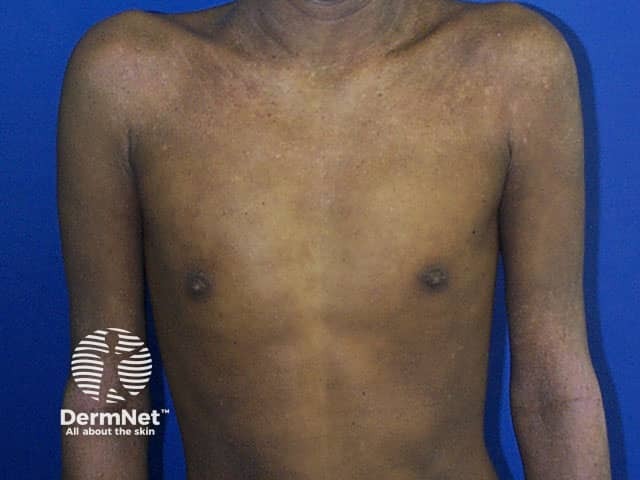
Atopic eczema
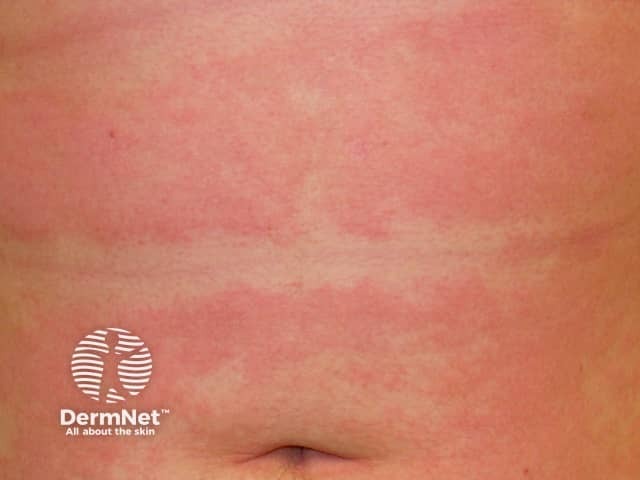
Subacute atopic dermatitis
There is some evidence supporting successful interval extension between maintenance doses.
Link to key clinical-trial evidence about dupilumab.
Most common (> 5%) adverse reactions associated with dupilumab treatment in clinical trials were:
Rarely, induction of another skin disorder has been reported, such as psoriasis. Dupilumab-associated inflammatory arthritis has also been reported.
Alcohol-induced facial erythema has been reported in those taking dupilumab.
Cutaneous T-cell lymphoma has been reported in 27 persons who recieved dupilumab. It may be that effective control of eczema has unmasked an underlying lymphoma, or that the original eruption was misdiagnosed as eczema. Affected individuals have been older (50-60 years), with extensive eczema (surface area exceeding 50%). Clues included failure of response or progression of the eruption despite dupilumab therapy, and the morphology of the eruption; these features should prompt consideration of biopsy.
No drug-specific blood-test monitoring is required.
Adverse effects involving the eye are wide-ranging in severity and frequency. Some studies suggest that dupilumab-associated ocular surface disease (DAOSD) may be greater in those with severe atopic dermatitis. These include:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).