Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Daniel Jun Yi Wong, Medical Student, University of Melbourne, 2012.
Introduction How it works How to use Efficacy Risks and side effects Effectiveness compared to steroids
Propranolol is a medicine from the class of beta-blockers. It has been used in the treatment of high blood pressure and other medical conditions for decades. Since 2008 it has also been used off-license for the treatment of complicated infantile haemangioma. The majority of infantile haemangiomas are not at risk of complication and do not require treatment.
Oral propranolol is available as tablets and liquid suspension. It has also been used as a 1% ointment. A similar medication, timolol, is available as a topical solution.
Propranolol is thought to inhibit the growth of blood vessels and constrict existing blood vessels within the haemangioma.[1] It acts on beta adrenergic receptors to decrease the release of blood vessel growth-signalling molecules (vascular endothelial growth factor and basic fibroblast growth factor) and by triggering programmed cell death.[2]
In many cases, haemangiomas are small and hidden; once they have reached their full size, they can be expected to slowly shrink over the next few years. These do not require treatment. However, propranolol should be considered when there is a risk of a complication. Indications for treatment include infantile haemangiomas that are growing in high risk sites where they may interfere with normal function such as breathing, feeding, vision and hearing, such as:
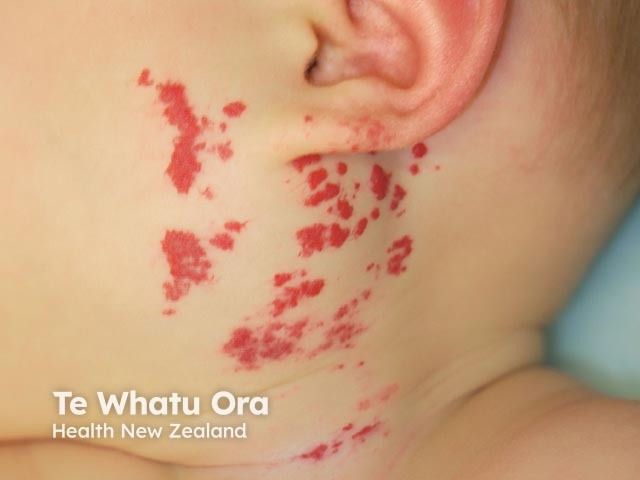
Haemangioma before propranolol
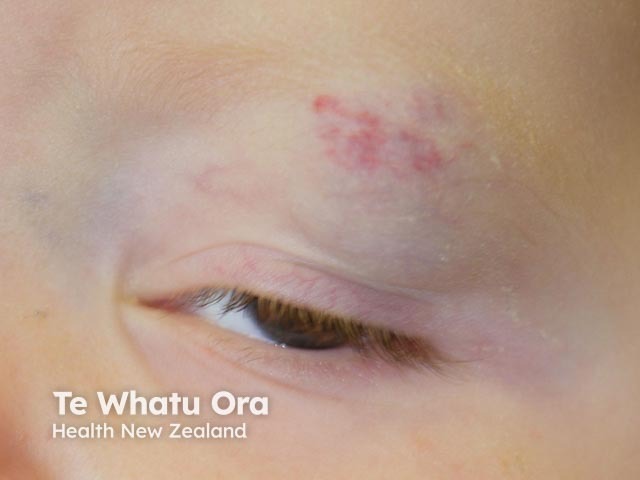
Haemangioma before propranolol
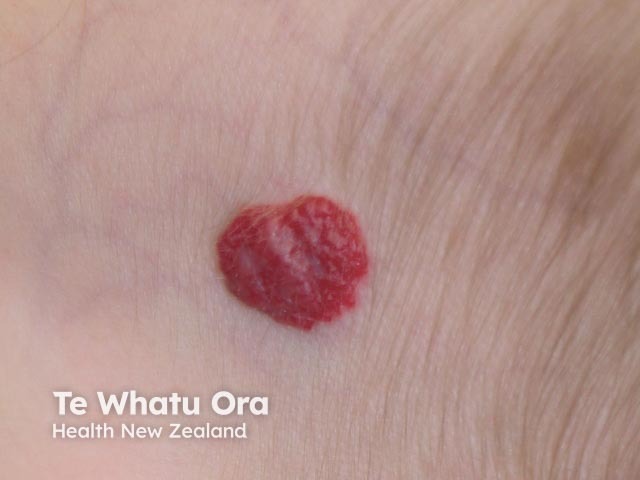
Haemangioma before propranolol
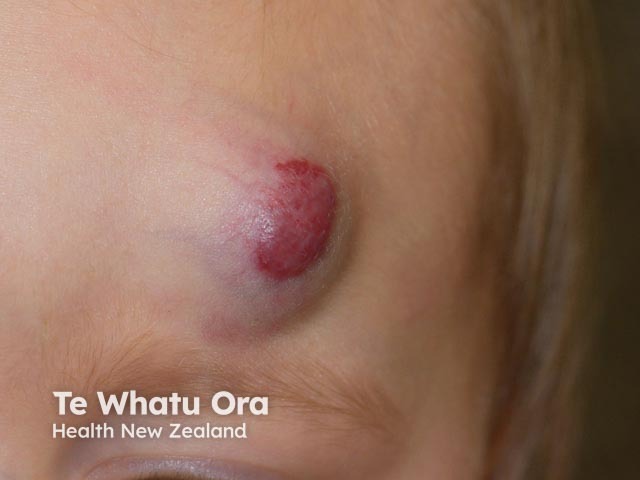
Haemangioma before propranolol
Propranolol is most effective when started during the growth phase of the haemangioma, in infants up to 6 months of age; they may begin to respond within 24 to 48 hours. The haemangioma softens (decrease in volume) and darkens in colour. The optimal duration of treatment is yet to be established, though most reports are of use for 3-12 months. Rebound growth may occur on cessation and gradual weaning may be required.
Propranolol may also be effective if it is started after the growth phase, even over the age of 12 months. It may speed up the rate at which the haemangioma decreases in size and eventually disappears. Some residual skin changes such as telangiectasia (dilated capillaries), scarring or fibrofatty residue may remain.[4]
For ulcerated infantile haemangiomas, propranolol shortens the time to healing and helps decrease pain. [5-6]
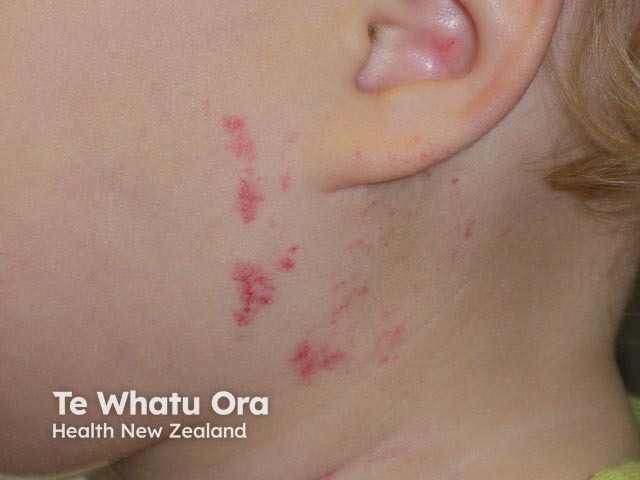
Haemangioma after propranolol
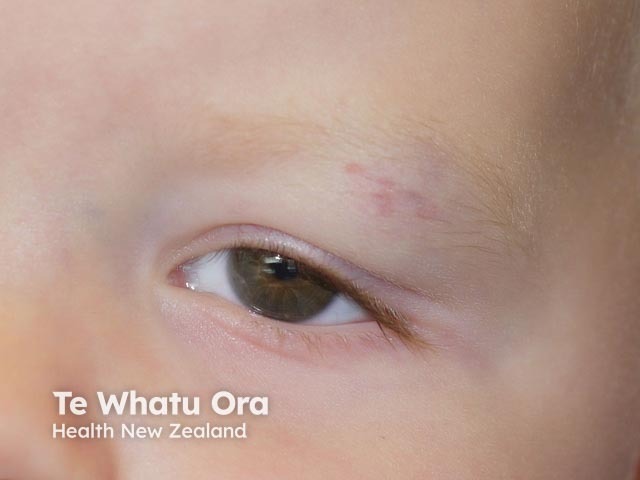
Haemangioma after propranolol
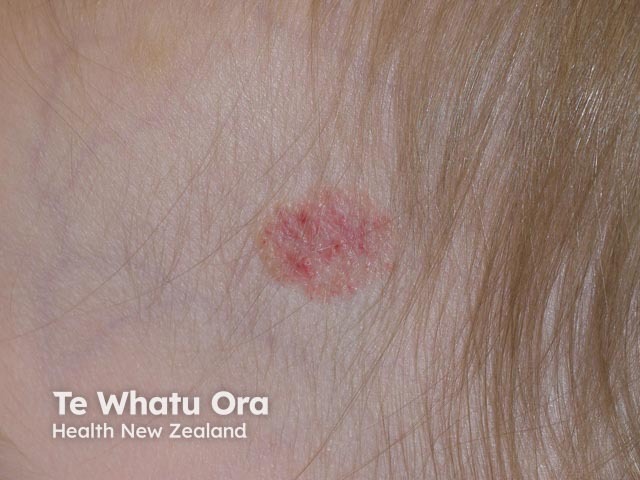
Haemangioma after propranolol
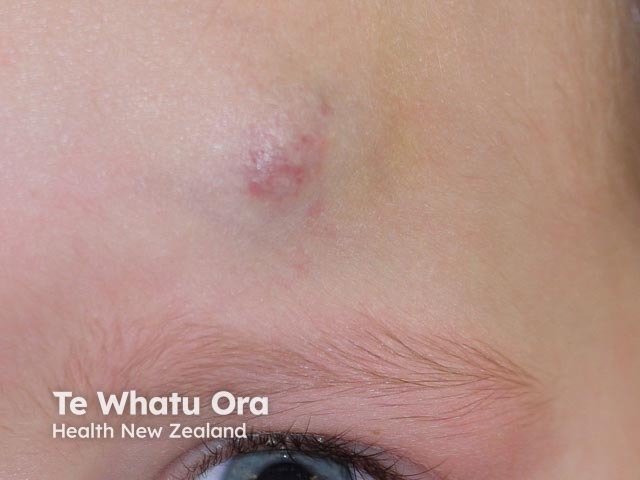
Haemangioma after propranolol
Propranolol is generally safe and well tolerated. Side effects are uncommon, and more likely in low birthweight babies, but may include:[7-8]
It is generally recommended when starting propranolol to monitor closely for cardiovascular effects and blood sugar.
Topical timolol is rarely associated with adverse effects but cardiovascular effects should be monitored especially in low birthweight infants.
Propranolol should be used with caution for children with multiple haemangiomas if there is involvement of blood vessels in the brain or heart. For example, PHACES syndrome, PELVIS syndrome or diffuse neonatal haemangiomatosis.
It is not always effective, so the haemangioma may persist and continue to grow larger.
Most dermatologists and other physicians now prefer to use propranolol as a first line treatment for those infantile haemangiomas that require treatment. There is increasing evidence that propranolol is safer and possibly more effective in treating infantile haemangioma compared to systemic steroid. Propranolol causes fewer side effects, less ulceration, and a lower requirement for surgery after treatment.[9] A consensus statement was prepared by the Australasian Vascular Anomalies Network and the Australasian Paediatric Dermatology Network (2017). [14] In summary:
Other treatments
Propranolol can be combined with pulsed dye laser (which treats superficial haemangioma).
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).