Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. First published December 2014; updated March 2018. Copy edited by Gus Mitchell/Maria McGivern.
This article was updated with financial support from Merck Sharp & Dohme (New Zealand) Limited, distributors of Keytruda® in New Zealand. Sponsorship does not influence content.
Introduction
The KEYNOTE-001 study
The KEYNOTE-002 study
The KEYNOTE-006 study
Conclusions
Pembrolizumab (Keytruda®) is a highly selective humanised monoclonal antibody directed against the programmed cell death 1 (PD-1) receptor on T cells. The drug blocks the PD-1 receptor, thereby preventing the formation of programmed cell death ligand 1 (PD-L1) complexes. This mechanism causes the activation of T-cell mediated immune responses against tumour cells.
On 4 September 2014, the US Food and Drug Administration (FDA) approved pembrolizumab as a breakthrough therapy for the treatment of metastatic melanoma, based on response rates demonstrated in clinical trial data from 173 patients with melanoma in a cohort of the KEYNOTE-001 study.
Since then results from two further clinical trials (KEYNOTE-002 and KEYNOTE-006) have continued to show the benefit of pembrolizumab for the treatment of advanced unresectable or metastatic melanoma.
Pembrolizumab is now registered for the treatment of advanced (metastatic or unresectable) melanoma in many countries worldwide, including Europe, the UK, and New Zealand.
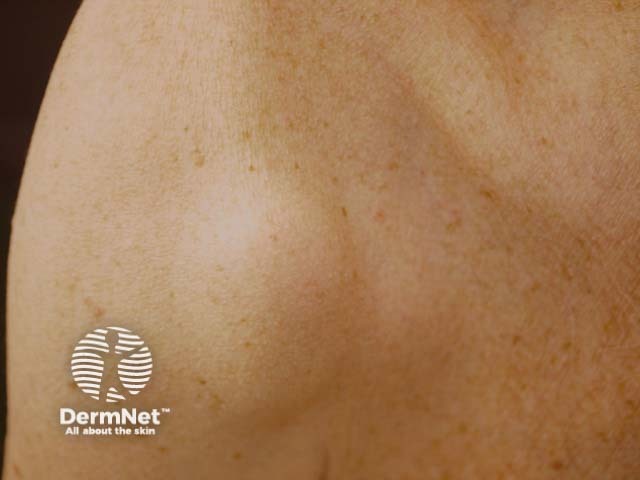
Subcutaneous metastatic melanoma
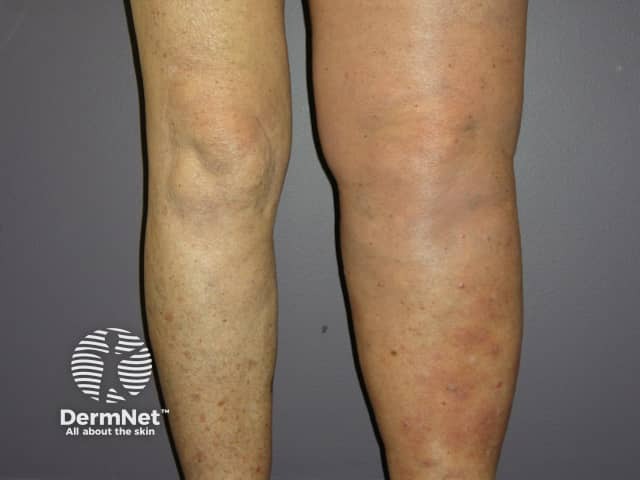
Metastatic melanoma
Table 1. Drug-related adverse reactions occurred in > 10% of patients in KEYNOTE-001
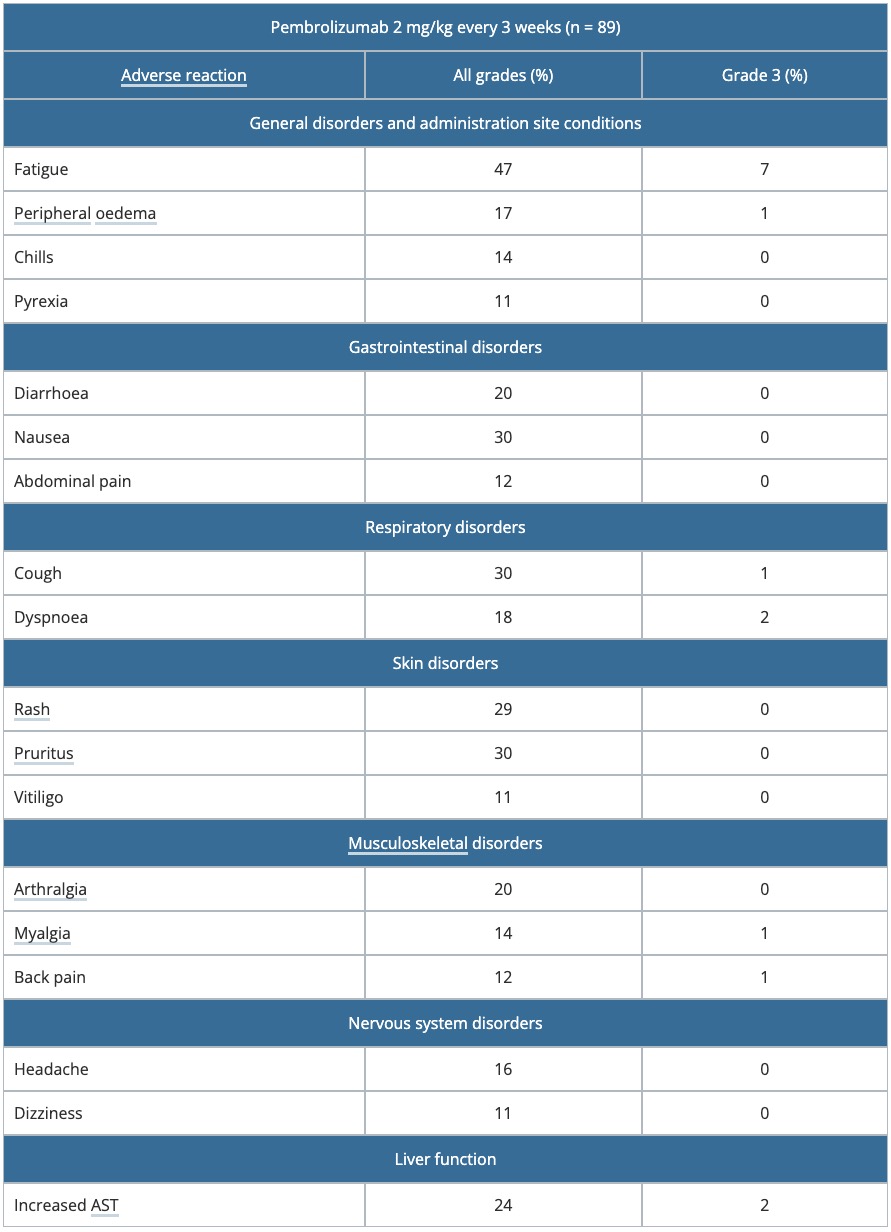
AST: aspartate transaminase
Table 2. Summary of efficacy parameters in KEYNOTE-002.
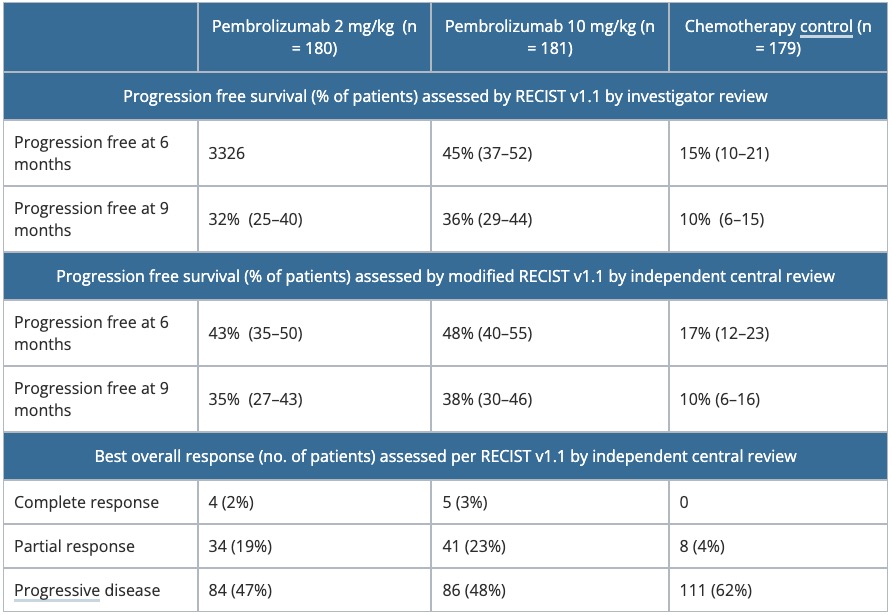
RECIST, Response Evaluation Criteria in Solid Tumors
Table 3. Summary of adverse events — KEYNOTE-002
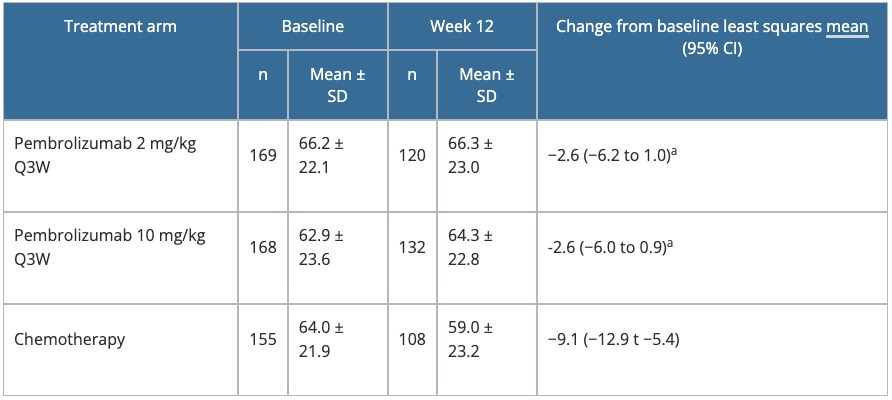
Table 4. Change from baseline to Week 12 in the GHS score of the EORTC QLQ-C30 in KEYNOTE-002
a P = 0.01 versus chemotherapy.
Table 5. Change from baseline to Week 12 in the GHS score of the EORTC QLQ-C30 — KEYNOTE-006
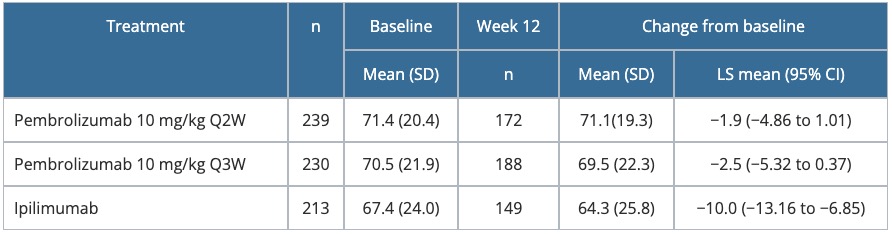
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30; GHS, Global Health Status; LS, least squares; Q2W, every 2 weeks; Q3W, every 3 weeks; SD, standard deviation
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).