Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: July, 2025
Author: Taylor Skinner (PharmD), Memorial University of Newfoundland, Canada (2025)
Peer reviewed by: Nancy Huang (MBChB), DermNet Medical Writer, New Zealand (2025)
Previous contributor: Dr Jacqueline K Nguyen, Resident, St Vincent's Hospital, Australia (2022)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
Contraindications
How it works
Dosage and administration
Benefits
Disadvantages
Adverse effects
Clascoterone 1% cream (Winlevi®) is a first-in-class topical androgen receptor inhibitor indicated for the treatment of acne vulgaris in patients aged 12 years and older.
Clascoterone first received approval from the US Food and Drug Administration (FDA) in 2020, followed by approvals in other regions such as Canada (2023), Australia (2024), New Zealand (2024), and the United Kingdom (2025).
The efficacy and safety of clascoterone 1% cream in acne vulgaris have been demonstrated in multiple clinical trials, leading to its inclusion in several acne treatment guidelines.
A 2024 systematic review and meta-analysis comparing recent topical treatments found no significant differences in efficacy between clascoterone, trifarotene, and tazarotene after 12 weeks of use for moderate-to-severe acne.
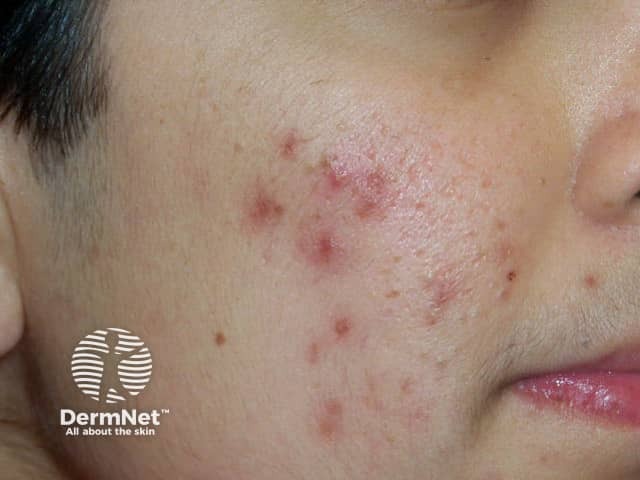
Comedonal and papulopustular acne suitable for clascoterone therapy
Clascoterone is under investigation in phase III clinical trials for the treatment of androgenetic alopecia (AGA) under the brand name Breezula®.
In phase II clinical trials of AGA, the clascoterone group showed significant clinical improvement compared to the vehicle group. These benefits are attributed to clascoterone’s antagonism of androgen receptors in scalp dermal papilla cells. Clascoterone is thought to reduce the production of prostaglandin D2 and interleukin 6 (IL-6), thereby regulating sebum secretion and mitigating hair miniaturisation.
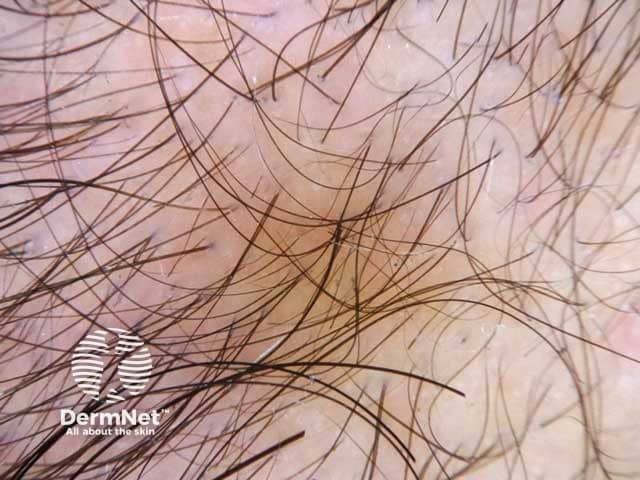
Hair shaft diameter variability in androgenetic alopecia (MPHL-patient1)
Clascoterone is contraindicated in anyone with a known hypersensitivity to the drug or its excipients.
The safety and efficacy of clascoterone have not been established in:
Though the exact mechanism is unclear, clascoterone exerts its effects through competitive antagonism of androgen receptors. Blocking the action of androgens (such as dihydrotestosterone) on sebocytes leads to a reduction in sebum production and inflammation, both of which are involved in acne pathogenesis.
Clascoterone is available as a 1% (10 mg/g) cream. It should be applied as a thin, uniform layer to the area affected by acne (rather than just individual spots) twice daily, in the morning and evening.
The majority of reported adverse effects are mild and infrequent.
Local effects may include:
Other reported adverse effects include:
Hypothalamic-pituitary-adrenal (HPA) axis suppression:
Hyperkalaemia (clinical trial finding of unknown significance):
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).