Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer, Auckland, NZ. Chief Editor: Dr Amanda Oakley, Dermatologist, NZ (2017). DermNet Update (2023).
Introduction How it works Dosage and administration Pregnancy, lactation, and geriatrics Adverse effects Evidence Other uses
In December 2016, the US Food and Drug Administration (FDA) approved crisaborole topical ointment, 2%, to treat mild to moderate atopic dermatitis in patients 2 years of age and older. The age limit in the US has subsequently been changed to include patients from 3 months of age.
Crisaborole 2% ointment has subsequently been approved for use in Australia and New Zealand (2019) on prescription for patients from 2 years of age with mild to moderate atopic dermatitis.
Crisaborole is a boron-based phosphodiesterase 4 inhibitor (PDE-4). It is a non-steroidal topical monotherapy that inhibits the phosphodiesterase (PDE)-4 enzyme in the skin. Overactive PDE-4 has been shown to contribute to the signs and symptoms of atopic dermatitis.
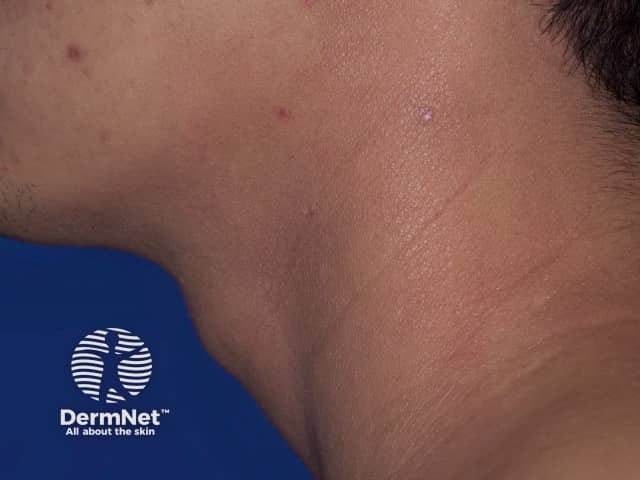
Atopic dermatitis of neck
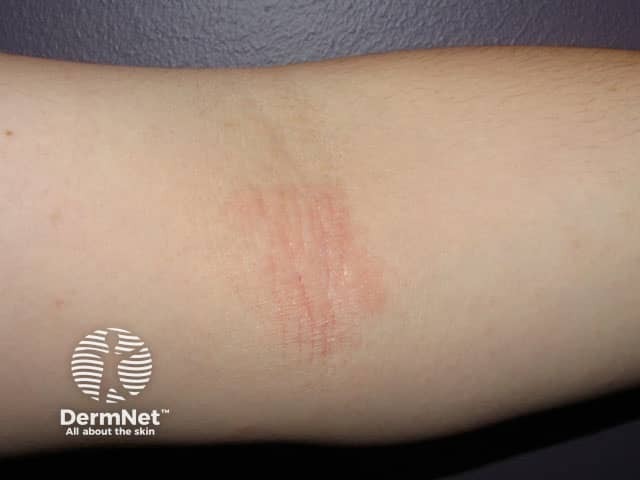
Atopic dermatitis
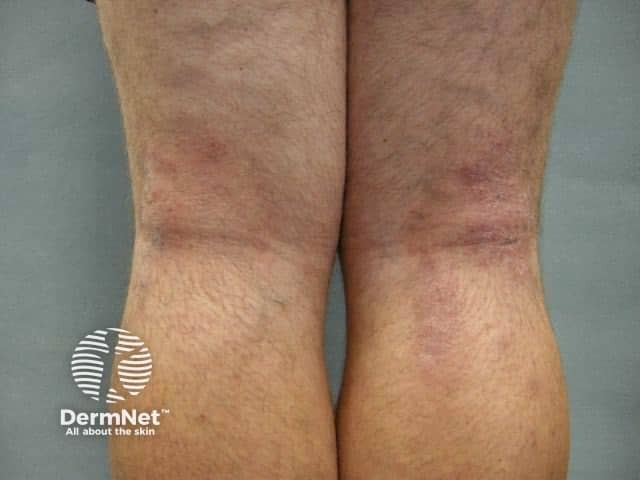
Atopic dermatitis
Crisaborole has been reported to be of benefit (but not yet approved) for intertriginous/flexural psoriasis and facial psoriasis, and chronic irritant contact dermatitis.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).