Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Heba Fawal, Department of Dermatology, Lattakia National Hospital, Lattakia, Syria. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. June 2020.
Introduction How it works Uses Side effects Monitoring Contraindications
Sodium stibogluconate is a medication used to treat cutaneous, visceral, and mucosal leishmaniasis, a parasitic infection transmitted by sand-fly bites. It is also known by its chemical name antimony D-gluconic acid, and the brand name Pentostam®.
Sodium stibogluconate is also occasionally used to treat acute myeloid leukaemia.
Sodium stibogluconate is not approved by the US Food and Drugs Administration and is not available in New Zealand. It is most used in tropical countries.
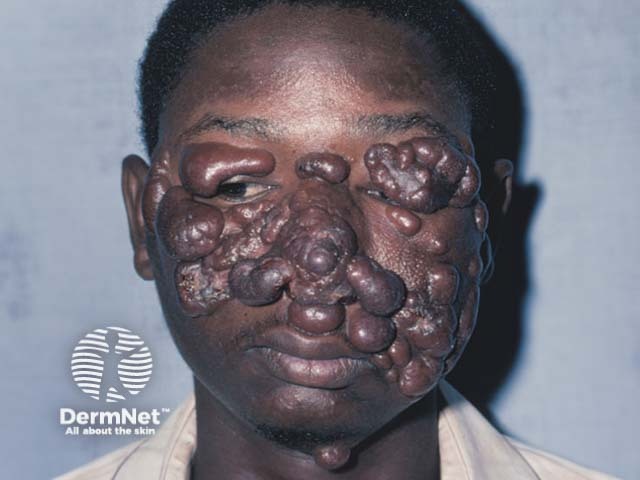
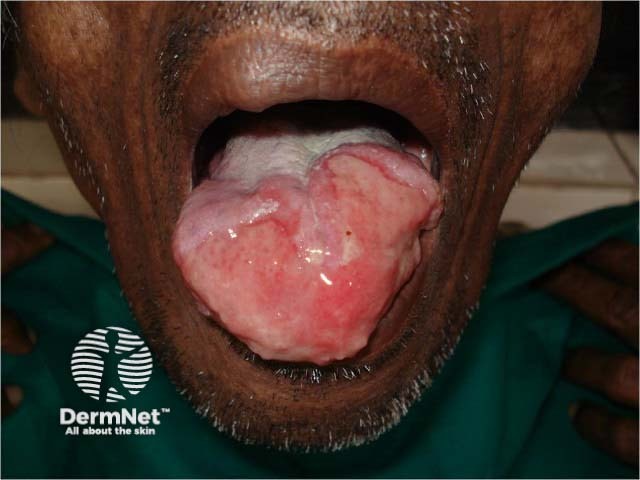
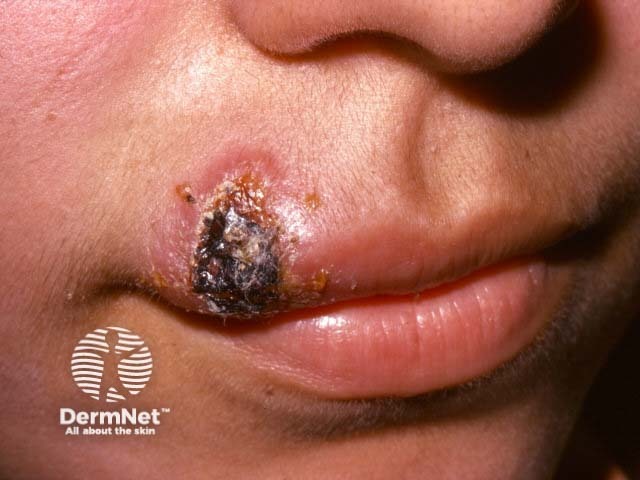
Sodium stibogluconate belongs to the class of medicines known as the pentavalent antimonials. Its mechanism is unclear; it may act by binding to thiol groups (–SH) in the parasite and inhibiting the formation of adenosine triphosphate (ATP) and guanosine triphosphate [GTP].
Sodium stibogluconate is eliminated by the kidneys and has a half-life of 6 hours.
Sodium stibogluconate is usually administered by intravenous or intramuscular injection daily at a dose of 20 mg/kg/day (maximum 850 mg) for 21–28 days.
Intralesional injections may be used for a single lesion or a few small lesions of cutaneous leishmaniasis. Intralesional injections are not suitable for lesions on the nose, ears, fingers, eyelids, lips, or genitalia. The intradermal injections are preceded by local anaesthetic or cryotherapy. The dose is 0.1 mL/cm2 (ie, 0.2–5 mL in up to 4–5 sites) repeated every 3–7 days until healing occurs.
Side effects and risks from sodium stibogluconate can be significant and are more likely to occur in older patients. They include:
Elevated serum amylase levels are seen very commonly with sodium stibogluconate administration and rapidly recover with cessation of treatment. Although symptoms are common, clinical pancreatitis is rarely severe, and fatal pancreatitis is extremely rare.
Acute hepatocellular damage and a loss of functional metabolic capacity of the liver is rapidly reversible on stopping treatment with sodium stibogluconate.
The following tests should be undertaken before treatment and weekly during treatment with sodium stibogluconate.
Adverse effects of intralesional injection of stibogluconate can include:
Contraindications to the use of sodium stibogluconate include:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).