Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author: Anoma Ranaweera, Staff Writer, 2012. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Updated June 2018. Copy edited by Gus Mitchell.
Introduction - Advanced BCC
Introduction
How to use
How it works
Potential drug interactions
Adverse events
Use in particular populations
Future considerations
Basal cell carcinoma (BCC) is usually curable if the lesions are restricted to a small area of the skin. However, in rare cases, the lesions can become disfiguring, invade surrounding tissue, or metastasise. In these instances of advanced BCC, the disease cannot be effectively treated with the standard treatments of surgery or radiation.
On January 30, 2012, the US Food and Drugs Administration (FDA) approved vismodegib (Erivedge®, made by Genentech, Inc. USA) for the treatment of adults with metastatic basal cell carcinoma. It is not currently funded by PHARMAC in New Zealand (Aug 2016).
Vismodegib is intended for use in adult patients with locally advanced basal cell cancer who are not candidates for surgery or radiation and patients whose cancer has metastasized.
This is the first FDA-approved drug for use in advanced forms of BCC, one of the most common skin cancers.
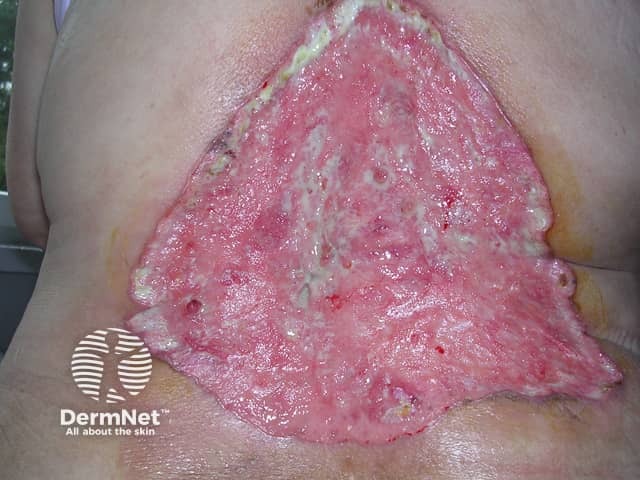
Advanced basal cell carcinoma
Link to key clinical-trial evidence about vismodegib
The most common adverse reactions (having an incidence of 10% or more) associated with vismodegib in clinical trials are:
The following adverse drug reactions have been identified during post-approval use of vismodegib based on case reports and investigator-initiated studies:
In a study in 60 healthy subjects, therapeutic doses of vismodegib did not have a significant effect on the QTc interval (a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle).
Roche and Genentech are currently evaluating vismodegib in a phase 2 trial for patients with operable forms of BCC.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).