Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet New Zealand Editor in Chief: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, August 2016. Copy editors: Gus Mitchell/Maria McGivern. October 2017.
Introduction Major trials supporting clinical efficacy of brodalumab Future potential
Brodalumab (Siliq™; Valeant Pharmaceuticals, New Jersey, USA) is a human monoclonal IgG2 antibody that selectively binds to human interleukin-17 (IL-17) receptor and inhibits its interactions with a number of cytokines of the IL–17 family. Brodalumab targets the receptor for IL–17, which is a cytokine or messenger protein involved in inflammation resulting in psoriasis.
In February 2017, brodalumab received US Food and Drug Administration (FDA) approval to treat psoriasis in adult patients who have not responded to other treatments. In May 2017, the European Medicines Agency's Committee for Medicinal Products for Human Use recommended the granting of a marketing authorisation for brodalumab for the treatment of moderate–to–severe plaque psoriasis in adults who are candidates for systemic therapy (treatment using substances that travel through the bloodstream, after being taken by mouth or injected) or phototherapy (ultraviolet radiation treatment).
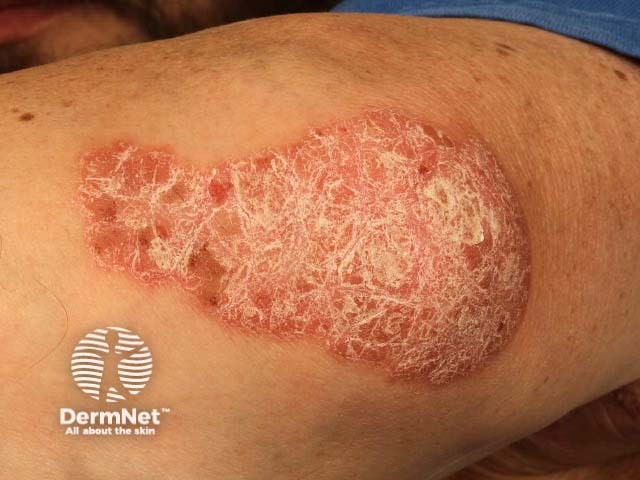
Chronic plaque psoriasis of elbow
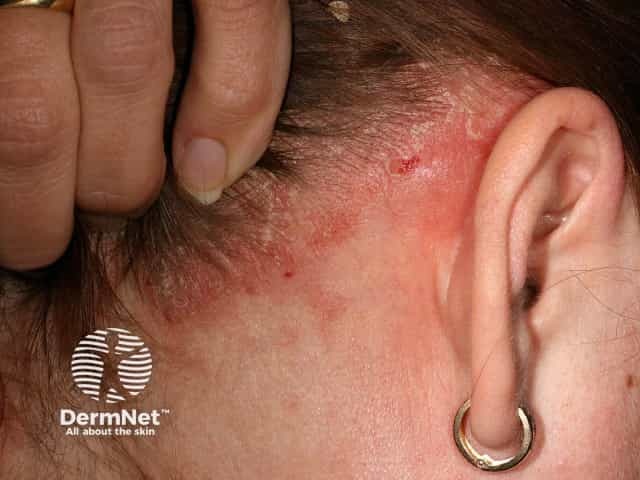
Scalp psoriasis
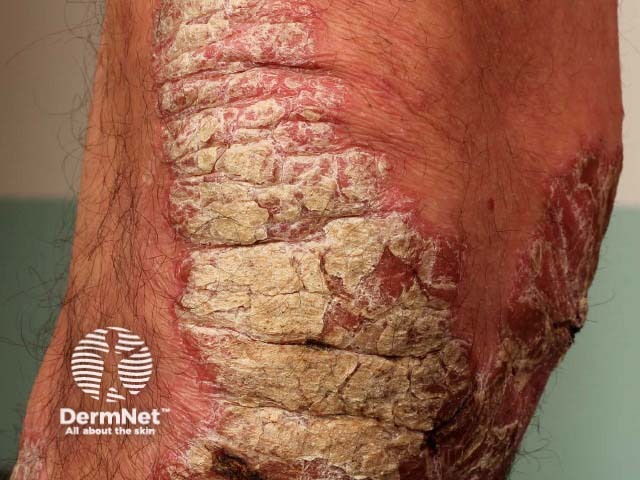
Chronic plaque psoriasis
The approval of brodalumab was based on three phase 3 trials:
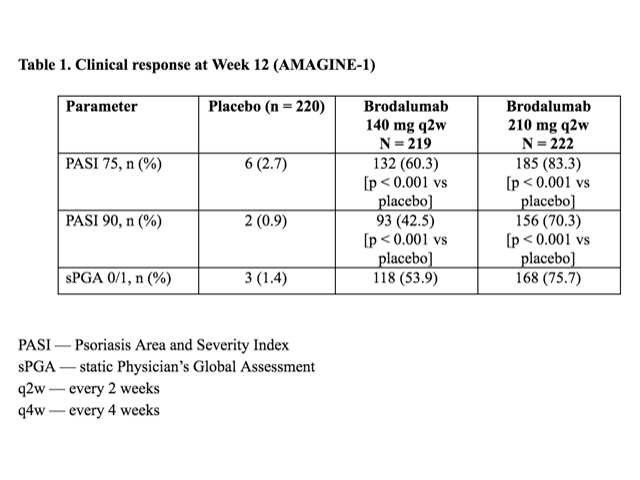
Table 1 – Clinical response at Week 12 (AMAGINE–1)
The most frequent adverse events (≥ 5% in any group) at Week 12 were:
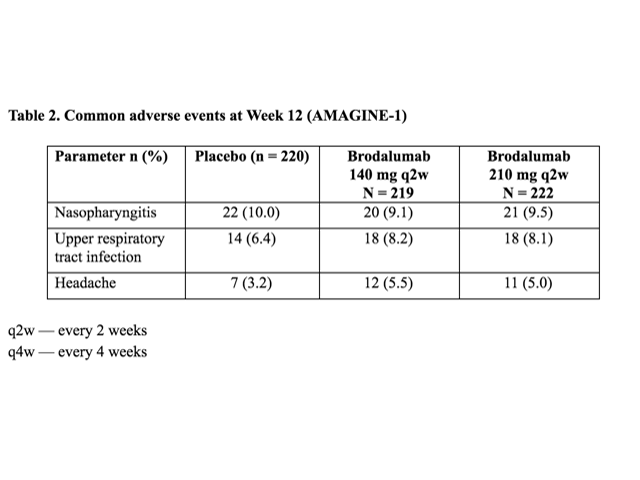
Table 2. Common adverse events at Week 12 (AMAGINE-1)
There were four fatal adverse events through Week 52: these included:
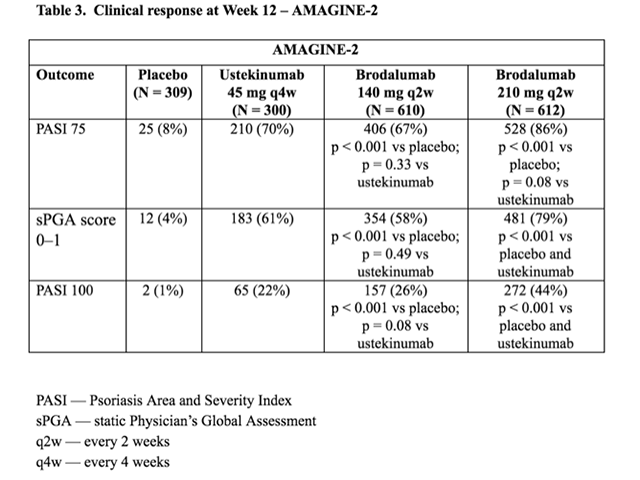
Table 3. Clinical response at Week 12 — AMAGINE–2
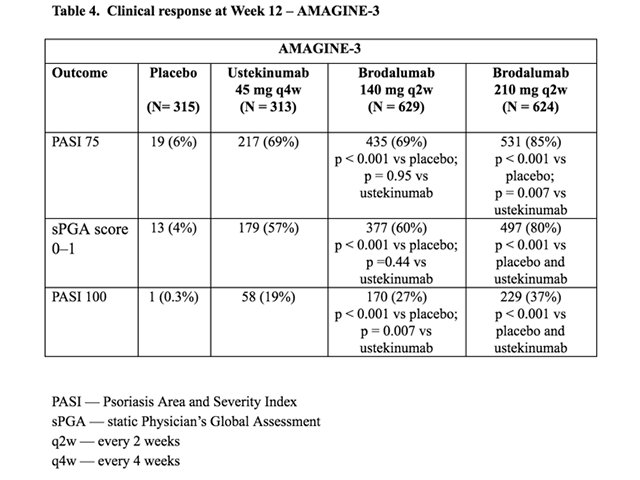
Table 4. Clinical response at Week 12 — AMAGINE–3
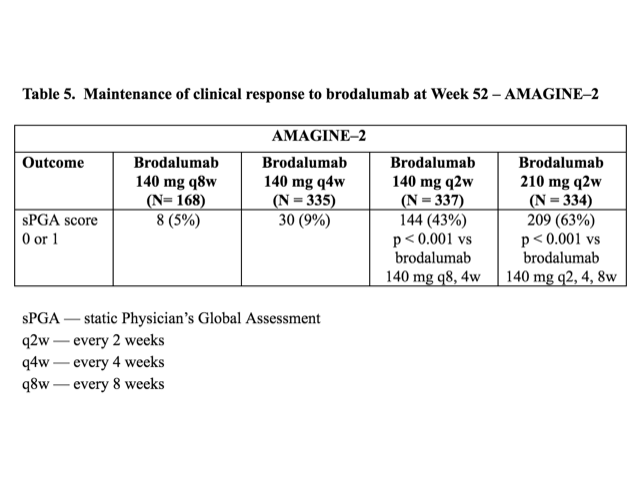
Table 5. Maintenance of clinical response to brodalumab at Week 52 — AMAGINE–2
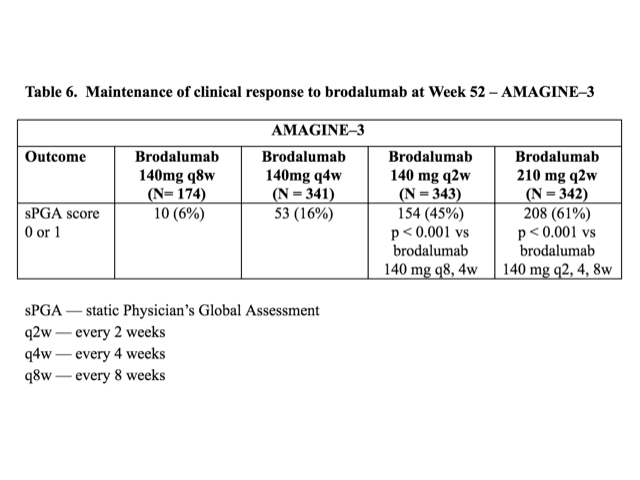
Table 6. Maintenance of clinical response to brodalumab at Week 52 — AMAGINE–3
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).