Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author: Anoma Ranaweera, Medical Writer. DermNet New Zealand Editor in Chief: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy editors: Gus Mitchell, Maria McGivern. July 2017.
Introduction Uses How it works How to use Use in specific populations Risks Outlook
In February 2017, the biological treatment brodalumab (Siliq™; Valeant Pharmaceuticals, New Jersey, USA) received US Food and Drug Administration (FDA) approval to treat psoriasis in adult patients who have not responded to other treatments.
Valeant expects to commence sales and marketing of brodalumab in the United States in the second half of 2017.
Because of the observed risk of suicidal ideation and behaviour, the labelling for brodalumab includes a boxed warning and the drug is only available through a restricted programme under the FDA’s Risk Evaluation and Mitigation Strategies (REMS) Program. Notable requirements of the programme are included below.
A causal association between treatment with brodalumab and the increased risk of suicidal ideation and behaviour has not been established.
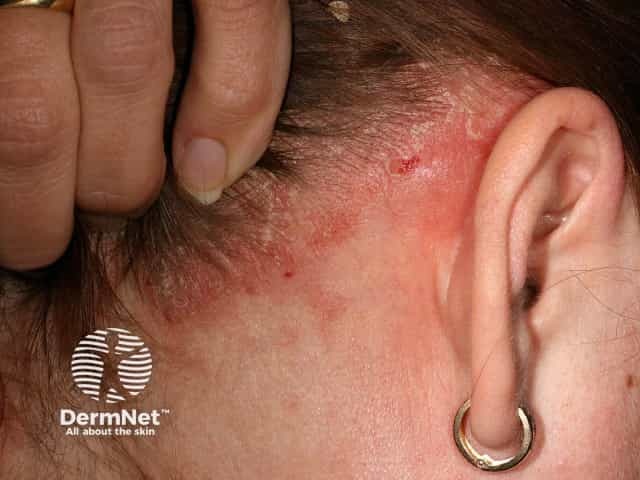
Scalp psoriasis
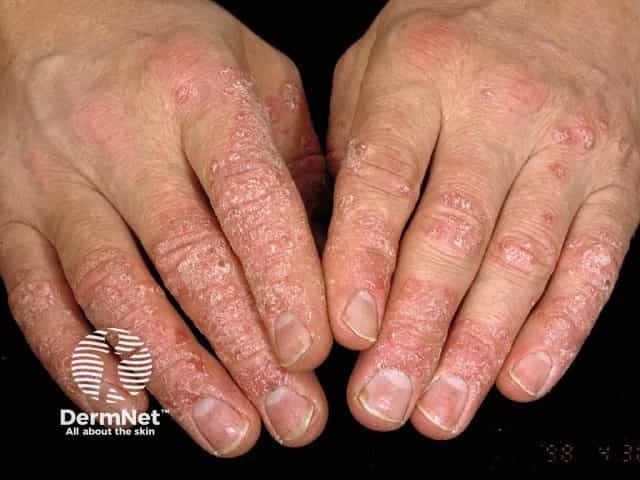
Psoriasis of hands
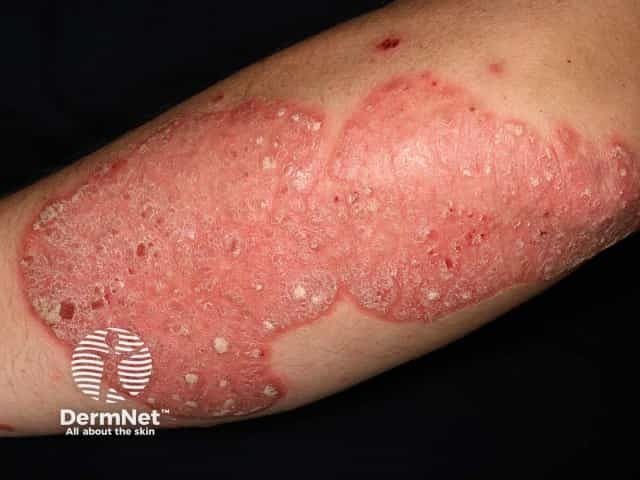
Chronic plaque psoriasis
Brodalumab is used for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy and have failed to respond, or have stopped responding to other systemic therapies.
It is recommended for candidates for whom systemic therapy or phototherapy have failed to provide results or adequate treatment.
Brodalumab is a monoclonal immunoglobulin G2 (IgG2) antibody that inhibits inflammatory reactions and selectively binds to the receptor of interleukin 17 (IL-17), a cytokine that initiates inflammation.
The IL-17 receptor is a protein expressed on the cell surface and is a component of receptor complexes used by multiple IL-17 family cytokines, such as IL-17A, IL-17F, IL-17C, IL-17A/F heterodimer, and IL-25. Blocking the IL-17 receptor inhibits IL-17 cytokine-induced responses, including the release of proinflammatory cytokines and chemokines associated with the pathogenesis of psoriasis.
Brodalumab is administered subcutaneously. Each prefilled syringe (1.5 mL) contains 210 mg of brodalumab and is for single-use only.
There are no data available on the use of brodalumab in pregnant women to inform physicians of a drug-associated risk for major birth defects and miscarriage.
No adverse developmental effects have been observed in pregnant monkeys after subcutaneous administration of brodalumab at up to 26 times the maximum recommended dose for humans. (See Safety of medicines taken during pregnancy for more information.)
There is no information on the presence of brodalumab in human milk or its effects on the breastfed infant. The risk–benefit potential should be considered when prescribing brodalumab to a lactating mother. (See Lactation and medications used in dermatology).
The safety and effectiveness of brodalumab have not been evaluated in paediatric patients.
Clinical studies with brodalumab did not include sufficient numbers of subjects aged 65 years and over to determine whether older people will respond differently from younger individuals.
No trials have been conducted to assess the use of brodalumab in patients with hepatic or renal impairment.
The most common adverse reactions (≥ 1% of patients) reported with brodalumab include:
The following serious adverse reactions have been reported in clinical trials in patients treated with brodalumab:
There are no data available on the ability of live or inactive vaccines to elicit an immune response in patients receiving treatment with brodalumab. Live vaccines should not be used during brodalumab treatment.
The effect of drugs metabolised via the hepatic cytochrome P450 enzymes (such as warfarin and ciclosporin) may be altered during concurrent administration with brodalumab. The modification of the dosages of these drugs should be considered.
Brodalumab is contraindicated in individuals who:
Brodalumab has shown an acceptable safety profile and good efficacy in the treatment of moderate-to-severe plaque psoriasis. It provides an important new therapy in the management of psoriasis where there remains a significant unmet need for new agents with novel mechanisms of action, rapid onset of effect, and improved and sustained total skin clearance while aiding greater patient adherence and minimising drug-specific safety concerns.
However, the current evidence for brodalumab is insufficient to confirm the maintenance of these results in the long term.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).