Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera B.V.Sc; PhD (Clinical Biochemistry, University of Liverpool, UK); Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, June 2016.
Introduction
Clinical trial experience
Adverse event results
Future directions
Ixekizumab (Taltz®) is a humanised monoclonal immunoglobulin G antibody developed by Eli Lilly and Company that has been approved in the USA (March 2016) and Europe (April 2016) as a treatment for plaque psoriasis.
Ixekizumab is a specific inhibitor of interleukin-17A (IL-17A), a pro-inflammatory cytokine that has a role in the development of several inflammatory conditions including psoriasis.
The efficacy of ixekizumab as a treatment for moderate to severe plaque psoriasis has been evaluated in three randomised placebo-controlled trials, UNCOVER-1, UNCOVER-2 and UNCOVER-3. UNCOVER-2 and UNCOVER-3 also compared ixekizumab to etanercept.
Uncover-1 was a prospective, double-blind, multicentre trial that consisted of 1296 patients randomly distributed in a 1:1:1 ratio to receive 80 mg ixekizumab every two weeks (Q2W), 80 mg ixekizumab every four weeks (Q4W), or placebo, respectively.
In UNCOVER-2, 1224 patients were randomly assigned to receive subcutaneous placebo (n=168), etanercept (n=358), or ixekizumab every 2 weeks (Q2W; n=351) or every 4 weeks (Q4W; n=347).
Patients in UNCOVER-3 were randomly assigned to receive placebo (n=193), etanercept (n=382), ixekizumab every 2 weeks (Q2W; n=385), or ixekizumab every 4 weeks (Q2W; n=386).
Table 1: Efficacy results at week 12 in evaluable adults with plaque psoriasis in trials 1, 2 and 3
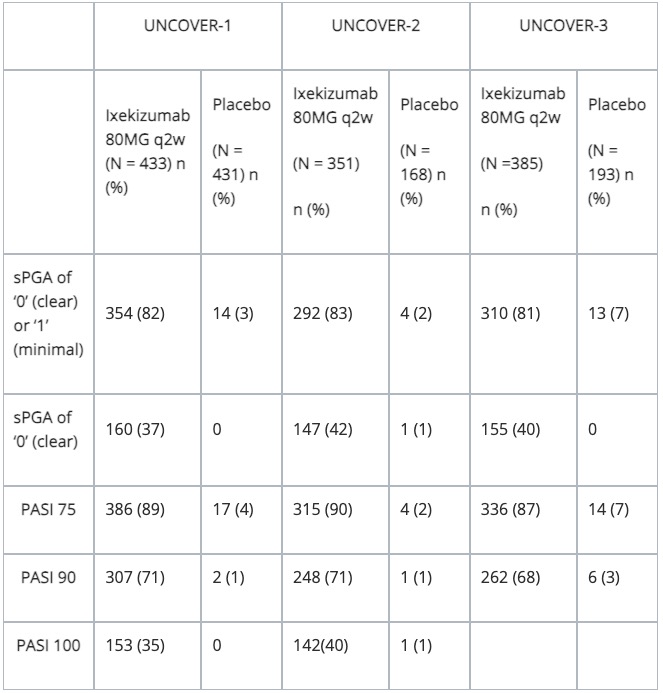
N = number of patients in the intent-to-treat population
Table 2 summarises the adverse reactions that occurred at a rate of ≥ 1% in the combined proportion of patients in the Ixekizumab group compared to etanercept and placebo during a 12-week treatment period.
Table 2: Adverse reactions in ≥1% of the TALTZ group vs placebo in adults with plaque psoriasis in trials 1, 2 and 3
Adverse Reactions |
Ixekizumab 80mg Q2W |
Etanercept |
Placebo |
Injection site reactions |
196 (17) |
32 (11) |
26 (3) |
Upper respiratory tract infections* |
163 (14) |
23 (8) |
101 (13) |
Nausea |
23 (2) |
1 (1) |
5 (1) |
Tinea infections |
17 (2) |
0 |
1 (1) |
*Upper respiratory tract infections cluster includes nasopharyngitis and rhinovirus infection.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).