Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author(s): Dr Claudia King, Southern Adelaide Local Health Network, Australia (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Contraindications
Benefits
Disadvantages
Side effects and risks
Use in special populations
Tapinarof is a topical aryl hydrocarbon receptor (AHR) agonist indicated for adults with plaque psoriasis.
Tapinarof (VTAMA®) is a once-daily novel topical agent for patients with mild, moderate, and severe plaque psoriasis, first approved by the US Food and Drug Administration (FDA) in May 2022. Phase II trials have indicated tapinarof is likely also effective for atopic dermatitis.
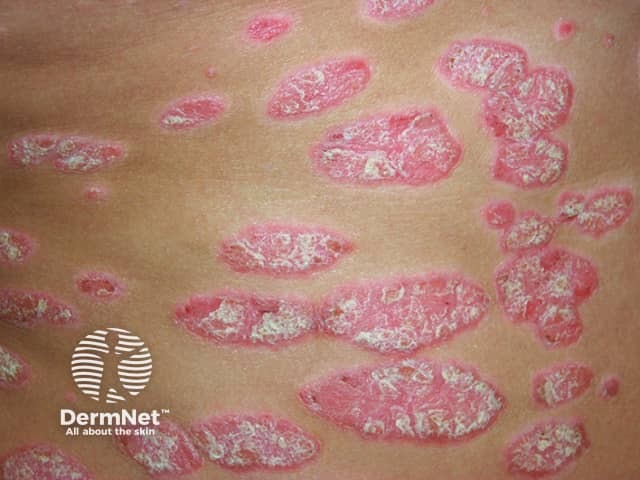
Chronic plaque psoriasis; topical treatment options would include tapinarof cream
More images of chronic plaque psoriasis
Tapinarof is a topical aryl hydrocarbon receptor (AHR) agonist. It binds and activates AHRs (a ligand-dependent transcription factor). This promotes:
Apart from hypersensitivity, there are no known contraindications.
Tapinarof 1% cream:
Tapinarof has demonstrated a reduction in the severity of mild, moderate, and severe plaque psoriasis in comparison to a control cream. During extended clinical trials, a significant proportion (~40%) of participants achieved disease clearance at least once compared to those receiving control cream (6%). With continued use, tapinarof can induce a prolonged remitting effective therapy (with a mean duration of 4 months remittance in extended trials).
Tapinarof has no known restriction on duration of treatment or extent of area treated; it exhibits low systemic absorption even in maximal use. It causes little to no irritation when used in sensitive skin areas and has overall been well tolerated in extended clinical trials.
Tapinarof provides an opportunity for steroid-sparing therapy for patients with plaque psoriasis.
Tapinarof has not yet been compared against pre-existing psoriasis treatments.
As it is newly approved, information on the risks of tapinarof are limited to pre-market clinical trials.
Most adverse events reported during clinical trials of tapinarof were mild or moderate.
Reported side-effects (with incidence) of tapinarof include:
Uncommon side-effects (<1%) included urticaria and drug eruptions.
Tapinarof was not associated with adverse effects during pregnancy in animal reproduction studies. There is currently insufficient data to establish risks in human pregnancy.
It is unknown whether tapinarof is expressed in human breast milk.
Tapinarof is under investigation for paediatric patients (aged 2–17) with plaque psoriasis.
No differences in safety or efficacy were observed in patients >65 years in PSOARING 1 and PSOARING 2 trials.
No dose adjustments are suggested in hepatic or renal impairment.
Images of chronic plaque psoriasis
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).