Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Sarah Seol, Waikato Hospital, New Zealand (2022)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Dosing and administration
Dosing considerations
Benefits
Side effects and risks
Contraindications
Tralokinumab is a fully human IgG4 monoclonal antibody used to treat moderate to severe atopic dermatitis.
In June 2021, the EU approved tralokinumab (Adtralza™) for the treatment of moderate to severe atopic dermatitis (AD) for adults in whom topical treatments are insufficient.
Tralokinumab specifically binds and neutralises the cytokine interleukin 13 (IL-13). This prevents the interaction with the IL-13 receptor and downstream inflammatory effects associated with atopic dermatitis.
It is produced using recombinant DNA technology in mouse myeloma cells.
Tralokinumab is used to treat atopic dermatitis.
Tralokinumab was also evaluated for use in several other conditions including severe asthma, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and ulcerative colitis (UC), however, trials were discontinued as limited benefits were observed.
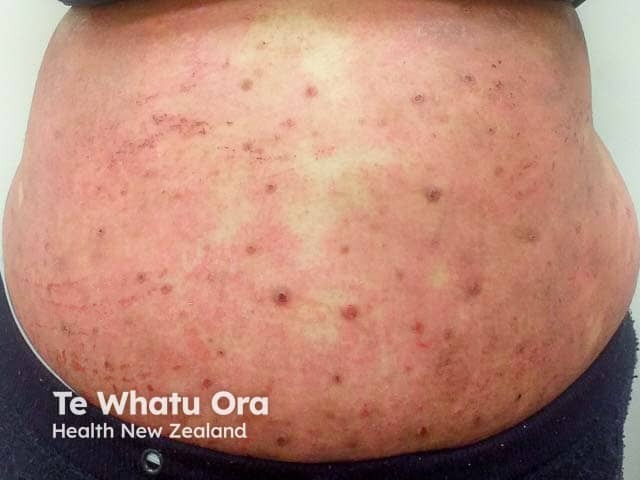
Extensive atopic dermatitis poorly controlled with emollients, potent topical steroids, and methotrexate - a potential candidate for an IL-13 antagonist
Atopic dermatitis is mainly caused by an immune response involving type 2 T-helper and lymphoid cells, and downstream IL-4, IL-5, and IL-13 cytokines. Recent studies suggest IL-13 to be the most abundant cytokine in atopic dermatitis skin lesions with its levels having a direct correlation to severity of disease.
Tralokinumab binds to and neutralises IL-13, thereby stopping the downstream inflammation and disruption of skin barrier seen in AD.
No dose adjustment in:
Increased dosing frequency:
Reduced dose in:
Limited data:
In two randomised, double-blinded, placebo-controlled, monotherapy studies (ECZTRA 1 and ECZTRA 2; 2020), and one study on the concomitant use of topical corticosteroids (ECZTRA 3; 2021), it was found that tralokinumab significantly improved patient-reported atopic dermatitis symptoms at 16 weeks, when compared to placebo.
Specifically, the number of patients achieving a 75% reduction in Eczema Area and Severity Index (EASI) score at 16 weeks, compared to control groups were as follows:
ECZTRA 1 also found a reduction in Staphylococcus aureus colonisation on lesional skin following tralokinumab treatment.
More common (>1%) adverse effects reported during tralokinumab use include:
Less common (<1%) reported adverse effects include:
Drug interaction studies with tralokinumab, specifically whether it changes the metabolism of Cytochrome P450 (CYP) substrates in adults with moderate to severe atopic dermatitis, are currently being conducted in the ECZTRA 4 trial.
Hypersensitivity to tralokinumab or any of the excipients including:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).