Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Genetic testing for melanoma — extra information
Lesions (cancerous) Diagnosis and testing
Genetic testing for melanoma
Author: Cathy Yunjia Zhao, Dermatology Registrar, Westmead Hospital, Sydney, New South Wales, Australia. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. October 2019.
Introduction Which are the melanoma germline gene mutations? More information What somatic genetic testing can be performed on the melanoma tumour? Bbenefits of germline genetic testing Disadvantages
What is genetic testing for melanoma?
Genes carry information in the form of DNA and control how cells function. A germline mutation affects all the cells in the body. A somatic mutation is restricted to a tumour cell, such as a melanoma cell.
Germline genetic testing for melanoma provides information on whether the patient has a mutation predisposing him/her to develop melanoma.
- About 10% of melanomas are secondary to gene mutations.
- The remaining 90% of melanomas are sporadic (unrelated to gene mutations).
If the patient carries a germline gene mutation for melanoma, they may have an increased risk of developing a melanoma. They may have a positive family history for melanoma, and the onset of melanoma may be at a younger age compared to sporadic melanoma.
A biopsy sample of melanoma can be tested in the laboratory for somatic mutations to give information on treatment options or prognosis.
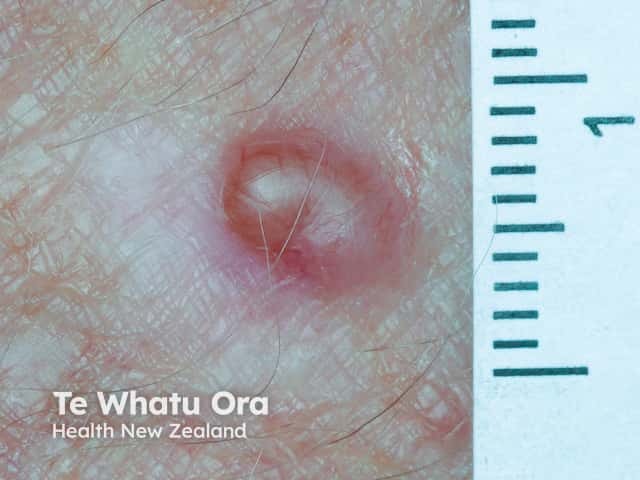
Amelanotic melanoma macro
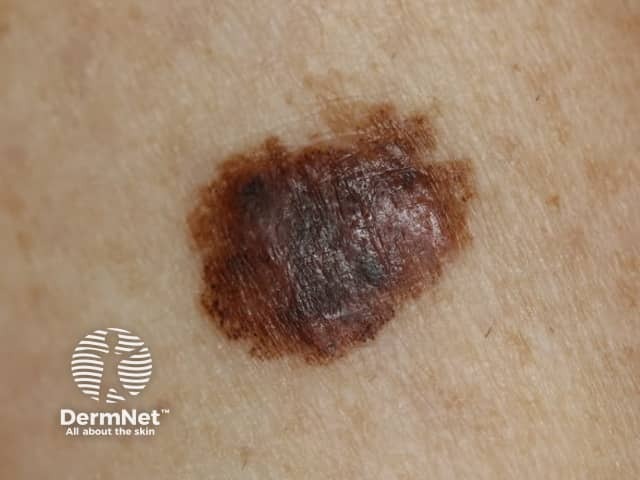
Large melanoma in situ
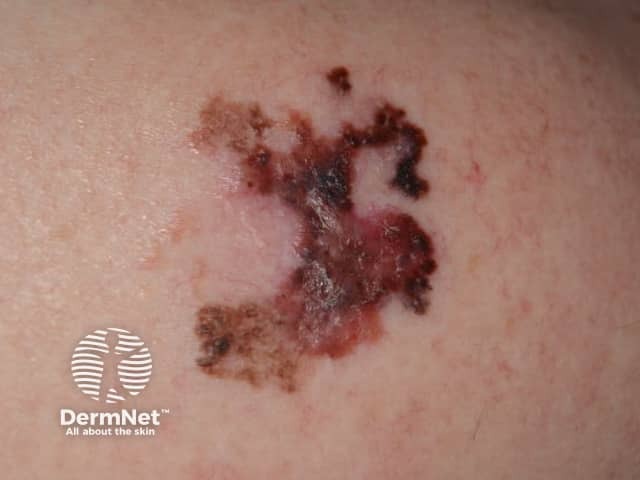
0.8 mm lentigo maligna melanoma
Which are the melanoma germline gene mutations?
Some of the germline gene mutations involved in cutaneous melanoma include, but are not limited to [1]:
- Cyclin-dependent kinase inhibitor 2A gene (CDKN2A)
- Cyclin-dependent kinase 4 gene (CDK4)
- Retinoblastoma gene (RB1)
- TERT promoter gene, microphthalmia-associated transcription factor gene (MITF) E318K
- BRCA1 associated protein 1 gene (BAP1)
- Microphthalmia-associated transcription factor (MITF)
- Tyrosinase (TYR) and tyrosinase-related protein 1 (TYRP1)
- Interferon regulatory factor 4 gene.
Features of cutaneous melanoma tumour syndromes may include:
- Early-onset of melanoma
- Multiple primary melanomas
- Members of one side of the family who have also had melanoma
- A family tendency to develop other rare malignancies [2].
CDKN2A mutation
CDKN2A mutation has been found in 1.2% of patients with a single primary melanoma and in 2.9% of patients with multiple primary melanomas. Carriers of CDKN2A mutation often develop cutaneous melanoma at a young age, have a positive family history of melanoma, and may also be predisposed to pancreatic cancer [3].
The American Academy of Dermatology’s guideline published in 2019 recommends that the following patients may warrant CDKN2A genetic testing where available [2]:
- A family history of invasive cutaneous melanoma or pancreatic cancer (3 or more affected members on one side of the family)
- Three or more primary invasive cutaneous melanomas, including one early-onset tumour (< 45 years of age).
BAP1 mutation
BAP1 mutation has been recognised as a rare cause of hereditary melanoma. Germline mutations of BAP1 are associated with:
- An increased risk of uveal melanoma
- Atypical Spitz tumours (also known as melanocytic BAP1-mutated atypical intradermal tumours [MBAITs])
- Cutaneous melanoma
- Renal cell carcinoma
- Mesothelioma.
Approximately 13% of carriers of a germline BAP1 mutation will develop a cutaneous melanoma, and a higher percentage of carriers will develop uveal melanoma [2,4].
The American Academy of Dermatology’s guideline published in 2019 recommends that the following history may warrant BAP1 genetic testing [2]:
- One or more atypical Spitz tumours and a family history of mesothelioma, meningioma, or uveal melanoma
- Two or more atypical Spitz tumours.
Tell me more about genetic testing for melanoma
Genetic counsellors and medical geneticists may arrange germline genetic testing for melanoma. During an initial genetic counselling appointment, the patient’s personal and family history will be thoroughly reviewed to estimate their inherited cancer risks. The patient will then be counselled regarding the benefits and disadvantages of performing the genetic test. The ultimate decision to pursue genetic testing for germline mutations is a complex decision based on family history, cancer patterns, patient wishes, and perceived risks versus benefits [2].
What somatic genetic testing can be performed on the melanoma tumour?
Oncogenic somatic mutations such as BRAF, NRAS, c-KIT or PTEN can be tested by immunohistochemistry or genomic techniques. Of these mutations, BRAF is the most common, observed in 50–70% of cutaneous melanomas. Information about an oncogenic mutation may help the clinician choose the best treatment for advanced melanoma, as there are targeted therapies such as BRAF-inhibitors for tumours harbouring certain BRAF mutations. Oncogenic mutation testing is recommended in patients with metastatic disease or if there is potential for enrolment into a relevant clinical trial [2].
Somatic genetic testing on a tumour may also provide prognostic information. Prognostic tests are mostly still undergoing investigation.
- TERT promoter mutations can be assessed for diagnostic and prognostic information [2].
- Gene expression profiling (GEP), also known as the 31-gene prognostic test, consists of a panel of 31 genes (28 signature genes plus 3 control genes). The GEP may be used to categorise melanoma into ‘high-risk’ and ‘low-risk’ tumours. High-risk patients are predicted to have a higher risk of distant melanoma metastases [5].
What are the benefits of germline genetic testing for melanoma?
Benefits to an individual include knowing their predisposition to melanoma, or to another type of cancer, depending on the gene tested [1].
- A positive test for melanoma indicates the patient’s skin should be monitored by an expert in early diagnosis of melanoma, which may include whole-body photographic skin surveillance and digital dermoscopy.
- A positive test may indicate the patient should be screened for other cancers. For example, a patient with a CDKN2A mutation may be regularly screened for pancreatic cancer.
- Genetic testing leads to a better understanding of melanoma by correlating certain gene mutations to melanoma phenotypes and identifying new drugs for patients.
What are the disadvantages of germline genetic testing for melanoma?
A positive test can increase patient concern about their personal and family's risk of developing cancer, and it may also lead to concern about obtaining health insurance or life insurance.
A negative test may give the patient a false sense of security, leading to reduced melanoma vigilance and sun protection. Therefore, it is important to inform the patient that approximately 90% of cutaneous melanoma are sporadic and unrelated to gene mutations.
References
- Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H. Hereditary melanoma: Update on syndromes and management: Emerging melanoma cancer complexes and genetic counseling. J Am Acad Dermatol. 2016 Mar;74(3):411–20; quiz 421-2. doi: 10.1016/j.jaad.2015.08.037. PMID: 26892651; PMCID: PMC4761106. PubMed. PubMed Central
- Swetter SM, Tsao H, Bichakjian CK et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019 Jan;80(1):208–50. doi: 10.1016/j.jaad.2018.08.055. Epub 2018 Nov 1. PMID: 30392755. PubMed
- Berwick M, Orlow I, Hummer AJ, et al. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1520–5. doi: 10.1158/1055-9965.EPI-06-0270. PMID: 16896043. PubMed. Journal
- Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013 Feb;45(2):116–26. doi: 10.1097/PAT.0b013e32835d0efb. PMID: 23277170. PubMed
- Klapperich ME, Bowen GM, Grossman D. Current controversies in early-stage melanoma: Questions on management and surveillance. J Am Acad Dermatol. 2019 Jan;80(1):15–25. doi: 10.1016/j.jaad.2018.03.054. PMID: 30553299. PubMed
On DermNet
- Melanoma
- Who gets melanoma?
- About melanoma: for patients
- Melanoma pathology
- Metastatic melanoma
- Genetics of melanoma – the detailed version
- Genes and melanoma – the simple version
- Melanocortin-1 receptor gene
- Interferon regulatory factor 4 gene
- Germline BAP1 mutation and BAP1 inactivated melanocytic tumours
- Blood-based melanoma detection
- Sun protection
Other websites
- Australian Melanoma Genome Project
- GenoMEL – the melanoma genetics consortium
- American Academy of Dermatology’s guidelines for the care of cutaneous melanoma
