Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. December 2019.
Introduction Key phase III trials with gel Key phase III trials with ointment
Calcipotriol/betamethasone dipropionate ointment and gel are topical medications for the treatment of plaque psoriasis [1–3]. The brand names of calcipotriol/betamethasone dipropionate are Daivobet® 50/500 ointment and Daivobet® 50/500 gel. Calcipotriol is called calcipotriene in some countries. An alternative brand name for the combination is Dovobet®.
Calcipotriol/betamethasone dipropionate ointment and gel are available in New Zealand with a doctor’s prescription and are fully funded by PHARMAC. The New Zealand marketing authorisation for Daivobet is held by LEO Pharma.
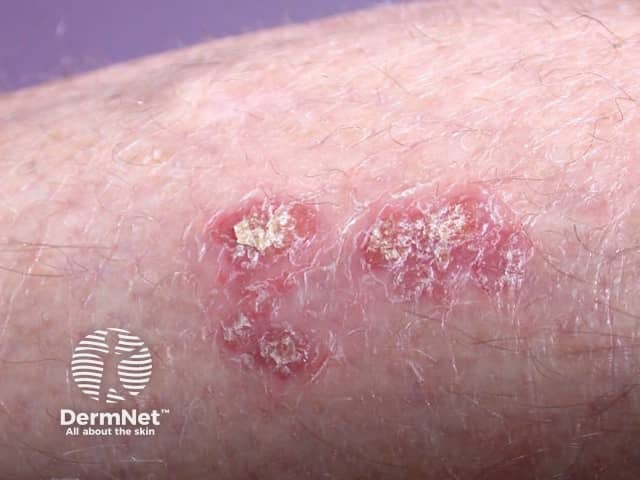
Chronic plaque psoriasis
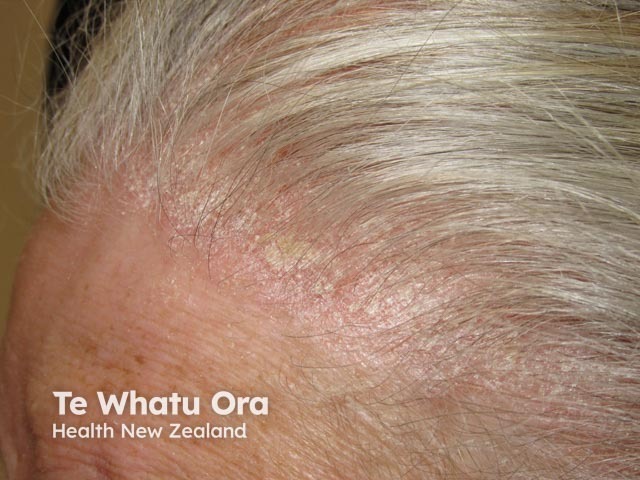
Scalp psoriasis often extends beyond the hair line
In an 8-week, multicentre, randomised, double-blind study, patients with scalp psoriasis were randomised to treatment with the two-compound scalp formulation (calcipotriol 50 mcg/g plus betamethasone 0.5 mg/g, as dipropionate) (n = 541), betamethasone 0.5 mg/g (as dipropionate) in the same vehicle (n = 556), calcipotriol 50 mcg/g in the same vehicle (n = 272) or vehicle alone (n = 136) [4].
A long-term (52-week) randomised, double-blind study in 869 patients with moderate-to-severe scalp psoriasis investigated the efficacy of the two-compound formulation (calcipotriol 50 mcg/g plus betamethasone dipropionate 0.5 mg/g) for scalp psoriasis in comparison to calcipotriol [5].
According to the Investigator's Global Assessment of disease severity, the number of patients who reached ‘satisfactorily controlled disease’ (‘absence of disease’, ‘mild disease’ or ‘very mild disease’) was higher in the two-compound group than in the calcipotriol group.
Several large-scale, real-life studies have assessed the efficacy of calcipotriol/betamethasone gel in plaque psoriasis of the body and scalp. Besides the demonstration of antipsoriatic efficacy in rigidly controlled clinical trials, confirmation of effectiveness in the real-life setting where factors such as patient preference, satisfaction, and adherence to treatment become increasingly relevant.
Pharmacoeconomic analyses have shown calcipotriene/betamethasone dipropionate gel formulations are more cost-effective than other topical therapies for psoriasis [9].
The primary objective was to investigate the safety of two treatment regimens involving the use of the two-compound calcipotriol/betamethasone dipropionate product over 52 weeks [5].
Six hundred and thirty-four patients were randomised double-blind to treatment (once daily, when required) with either:
Results
The calcipotriol/betamethasone combination was more effective and had a more rapid onset of action than either active constituent used alone, and was well tolerated. It is safe to transfer patients from betamethasone/calcipotriol to calcipotriol alone, with the maintenance of clinical effect.
A cost-utility analysis from a Swedish healthcare payer’s perspective used a decision-tree model with a 12-week time horizon to evaluate the cost-effectiveness of calcipotriol/betamethasone foam versus calcipotriol/betamethasone ointment [8].
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).