Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: November, 2022
Author: Nataki Duncan, MHS, MPH, Meharry Medical College, Tennessee, US.
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
How it works
Uses
Dosage and administration
Benefits
Use in specific populations
Adverse effects
Contraindications
Nemolizumab is a biological therapy used to treat moderate to severe prurigo nodularis (also called nodular prurigo) and atopic dermatitis.
Nemolizumab received its first approval in Japan in 2022 (Mitchga®) for those aged 13 years and over to treat severe atopic dermatitis.
In 2019, the U.S. Food and Drug Administration (FDA) awarded Galderma with a Breakthrough Therapy designation to investigate nemolizumab in treating pruritus associated with prurigo nodularis. Studies on efficacy and safety are currently underway.
Nemolizumab is a monoclonal antibody that acts as an interleukin 31 (IL-31) antagonist. Clinical trials have shown that nemolizumab effectively disrupts the itch-scratch cycle in chronic pruritic skin conditions.
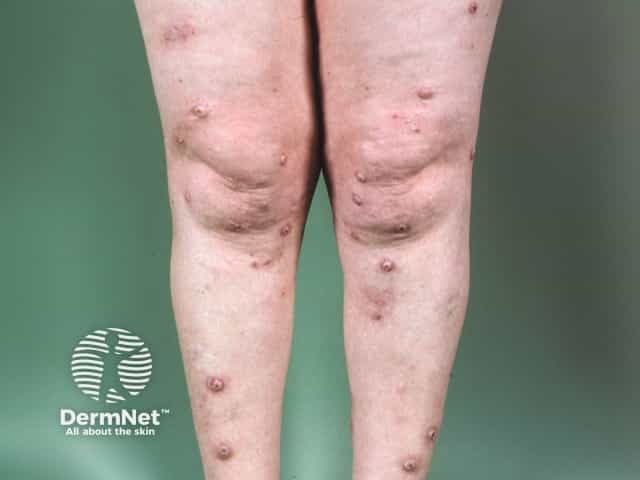
Nodular prurigo on the shins
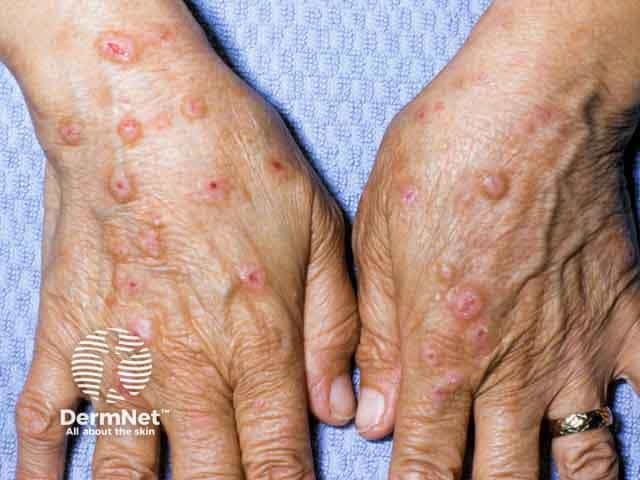
Prurigo on the dorsal hands in skin of colour
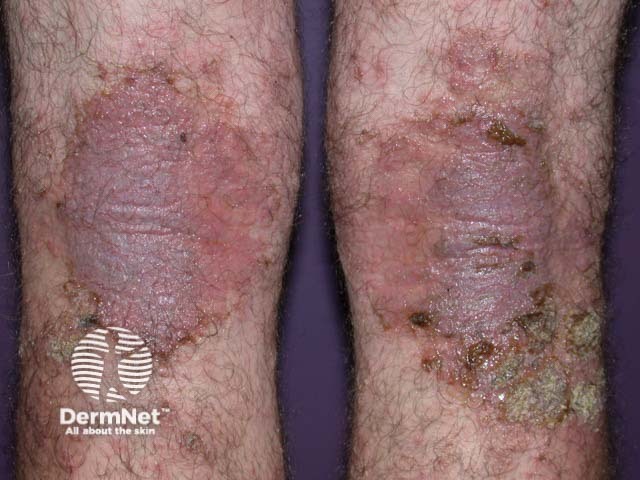
Infected atopic dermatitis
Nemolizumab is administered as a subcutaneous injection.
The dose approved in Japan for atopic dermatitis treatment is 60mg subcutaneous injection administered at intervals of four weeks.
In an open-label phase II clinical trial (Sidbury et al, 2022), 20 adolescent patients (mean age 14.8 years) with moderate to severe atopic dermatitis received a loading dose of 60 mg nemolizumab and a maintenance dose of 30 mg four weekly for 12 weeks, along with background use of topical corticosteroids or topical calcineurin inhibitors.
In a 68-week, randomised, placebo-controlled, phase III trial (Igarashi et al, 2024), 45 paediatric patients aged 6-12 years with AD received 30mg of nemolizumab every 4 weeks. It was found to be clinically effective and tolerable.
Patients with chronic kidney disease may experience severe uraemic pruritus. The pathogenesis of uraemic pruritus is unknown, but interleukin 31 (IL-31) is suspected to be involved.
A phase II trial (Kinugasa et al, 2021) investigated the efficacy and safety of nemolizumab in treating uraemic pruritus in hemodialysis patients. Patients received a one-time subcutaneous injection of nemolizumab (0.125, 0.5, or 2.0 mg/kg), control, or nalfurafine hydrochloride (NAL).
Nemolizumab generally had a good safety and tolerability profile compared to control or placebo groups. A 64-week extension study found no new safety concerns.
Adverse events reported in clinical trials include:
Serious adverse events that occurred in patients taking nemolizumab in clinical trials accounted for less than 5% of reported adverse events, and included:
Ongoing phase III trials will further explore the safety and efficacy of nemolizumab in treating prurigo nodularis and atopic dermatitis.
Currently, there are no formal drug interaction studies associated with nemolizumab.
See also immunisation in immunosuppressed dermatology patients for information on vaccination.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).