Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author(s): Jacob Junior Kang, The University of Auckland, New Zealand; Dr Libby Whittaker, DermNet Medical Writer, New Zealand (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Contraindications
Dosing and administration
Benefits
Disadvantages
Side-effects
Drug interactions
Use in specific populations
Talimogene laherparepvec (T-VEC) is a medication comprised of a genetically engineered attenuated strain of herpes simplex virus 1 with anti-tumour immune properties, used to treat melanoma.
T-VEC (brand name Imlygic®, previously OncoVEXGM-CSF) is one of the first globally approved oncolytic immunotherapeutic medications. It was approved by the United States Food and Drug Administration (FDA) and also by the European Medicines Agency (EMA) in 2015.
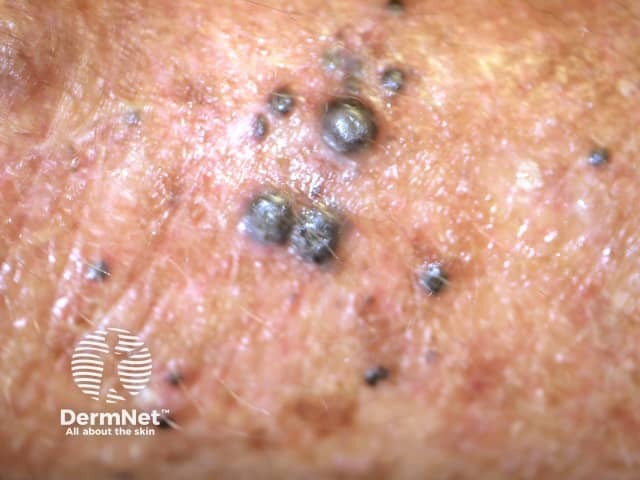
Multiple cutaneous metastasis from a melanoma, a potential candidate for talimogene
Talimogene laherparepvec (T-VEC) is predominantly used to treat unresectable metastatic melanoma in adult patients with no bone, brain, lung, or other visceral metastatic diseases.
Early-stage clinical trials are testing whether T-VEC may also be effective in treating:
Talimogene laherparepvec (T-VEC) is an oncolytic viral immunotherapy derived from Herpes simplex virus 1 (HSV-1).
It has been genetically modified (including deletions of ICP34.5 and ICP47) to selectively replicate within tumours and produce granulocyte-macrophage colony-stimulating factor (GM-CSF), causing tumour cell death and the release of tumour-derived antigens. This promotes an effector T-cell and systemic anti-tumour immune response.
Talimogene laherparepvec (T-VEC) is given via intralesional injection. Dosing is dependent on lesion size and treatment phase.
2 concentrations:
Dose:
Use up to 4mL total across all lesions per visit.
Treatment should be continued for at least 6 months, except if:
In the OPTiM study, T-VEC showed a higher durable response rate when compared with GM-CSF intralesional injection (16.3% vs 2.1% respectively) and overall response rate (26.4% vs. 5.7% respectively) in patients with stage IIIB, IIIC, and IVM1a melanoma.
T-VEC has also demonstrated potential systemic immune effects, by reducing the size of the following lesions by at least half:
T-VEC is generally well tolerated and has a good safety profile (see more below).
Common side-effects of T-VEC include:
Other reported adverse effects include rash, cellulitis (~2%), herpetic infections (including oral herpes), and a number of immune-mediated events such as worsening psoriasis, vasculitis, glomerulonephritis, and vitiligo.
Patients should be advised to seek medical attention if the following symptoms of potential herpes infection develop, such as:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).