Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Created 2008.
This section on dermatoscopic-histologic correlation is not intended to be a comprehensive overview of dermatoscopic diagnosis, which is covered elsewhere on this site. The terminology used is, however, similar and is based on the monograph by Kittler H et al.[1], on modified pattern analysis. Unless otherwise indicated, the diagrams in this section are reproduced from that monograph with the kind permission of the authors.
There is a close correlation between the patterns and features observed dermatoscopically and the histological features of biopsied or excised skin lesions, particularly in the case of pigmented lesions. With experience it is possible to predict the features that will be observed histopathologically based on the dermatoscopic examination. This correlation is important for both clinician and pathologist to understand as the two diagnostic modalities provide complementary information when used in the diagnosis of pigmented skin lesions [2]. This reflects the different ways in which they view the same lesion. Dermatoscopy provides an overview of the lesion viewed parallel to the skin surface, akin to an aerial photograph of a landscape, whereas histopathology provides a detailed assessment of a small part of the lesion that is viewed perpendicular to the skin surface, akin to a photograph from ground level of a hillside within the overall landscape. Correlation is essential to ensure that the pathologist has viewed the parts of a lesion in which specific dermatoscopic features of interest or concern have been noted. Using the analogy above one may not appreciate from a ground level photograph what is beyond the hill you are photographing whereas this may be obvious in an aerial photograph.
It is essential, therefore, that any pathology report issued on a skin lesion on which dermatoscopy has been performed should detail the histologic correlate of the dermatoscopic features noted. It is equally important that a dermatoscopist should be satisfied that the pathologist has provided an adequate explanation for the features they observed. If there is any doubt a phone call to clarify the situation and perhaps examination of deeper levels by the pathologist is indicated.
The dermatoscopic assessment of pigmented lesions using pattern analysis involves assessment of colour and pattern and the identification of clues to a specific diagnosis. Colour and pattern are used to assess the symmetry of a lesion. Each of these components of the assessment correlates with the histopathology.
Colours: The colours observed dermatoscopically correlate with the nature of the pigment / substance and, in the case of melanin, the depth of the pigment observed histopathologically (Figure 1).
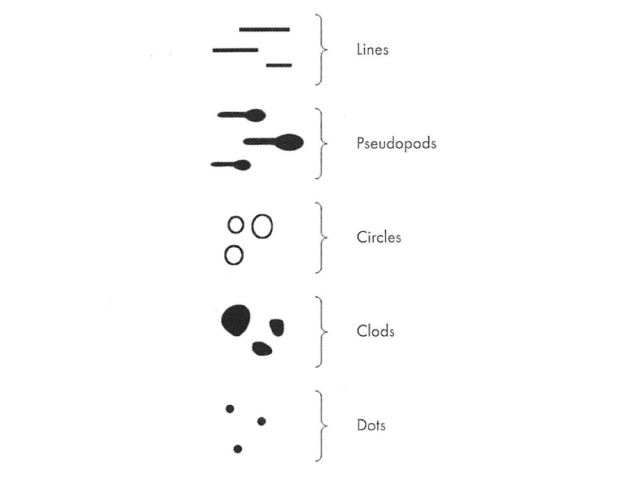
Elements: Dermatoscopic patterns are formed by the repetition of, or absence of a repetitive pattern of, one of five basic elements. These are lines, pseudopods, circles, clods and dots (Figure 2). The absence of a dominant repetitive pattern of one of these elements typifies the structureless pattern. You can click on the images for a larger view.
Figure 1 Figure 2 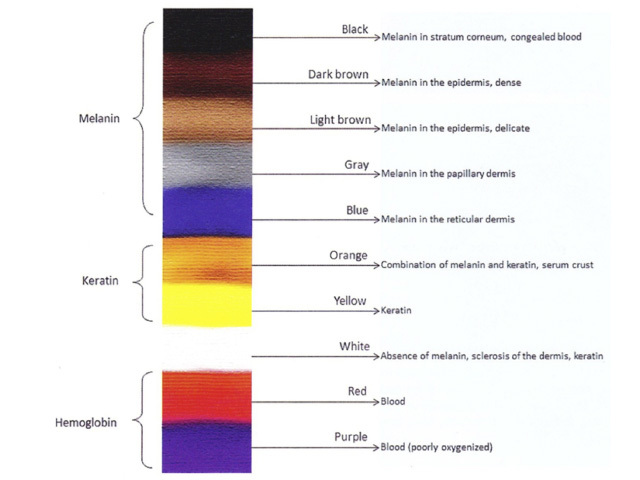
There are five dermatoscopic patterns of lines. These are reticular, branched, parallel, radial and curved (Figure 3).
Figure 3 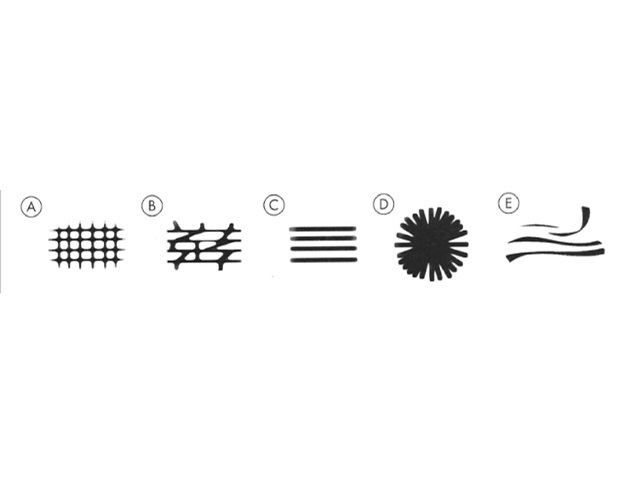
Figure 3. Diagram. Five possible patterns of lines. From Kittler et al [1].
Apart from radial lines, dermatoscopic patterns of lines reflect either the normal interdigitation of epidermal rete ridges (downgrowths) and dermal papillae (upward indentations, Figure 4a ); exaggerations, caused by acanthotic keratinocytic proliferations (such as seborrhoeic keratosis); or distortions of that pattern (such as broadened network due to exuberant melanocytic proliferation expanding epidermal rete ridges). Site specific variations in the anatomy of the dermoepidermal junction account for certain patterns, such as parallel lines on acral sites, or variations in the patterns seen in similar lesions in different anatomic sites.
Figure 4a 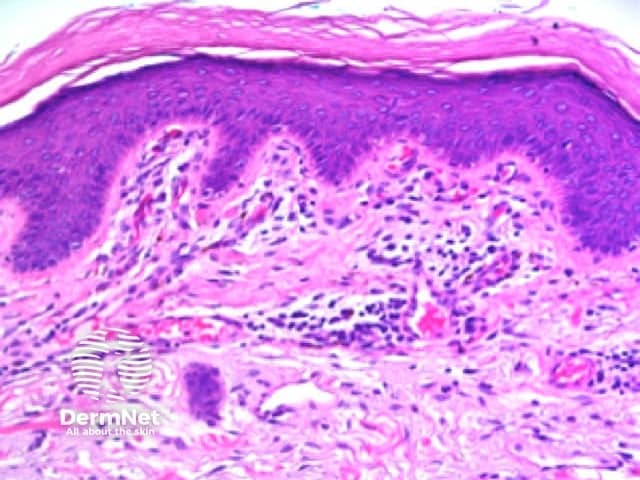
Radial lines
Radial lines are the dermatoscopic manifestation of peripheral extensions of pigmented lesional cells such as pigmented basal cell carcinoma or elongate nests of pigmented melanocytes in melanocytic proliferations. As radial lines are viewed parallel to the epidermal surface they are not evident in basal cell carcinoma sectioned perpendicular to the epidermal surface for histolological examination, precluding specific correlation. In melanocytic lesions they comprise elongate nests of melanocytes similar to pseudopods on histology. As such, the histologic correlation of radial lines will not be discussed further.
Reticular and branched lines
Reticular lines are straight and intersect at regular intervals at right angles. Branched lines are also straight but intersect at irregular intervals and not at right angles. The distinction is not always clear cut and rarely of great importance. These dermatoscopic patterns are seen in lesions in which there is basal epidermal hyperpigmentation and / or a proliferation of pigmented melanocytes preferentially involving the epidermal rete ridges with preservation of the epidermal architecture (Figure 5).
Figure 5 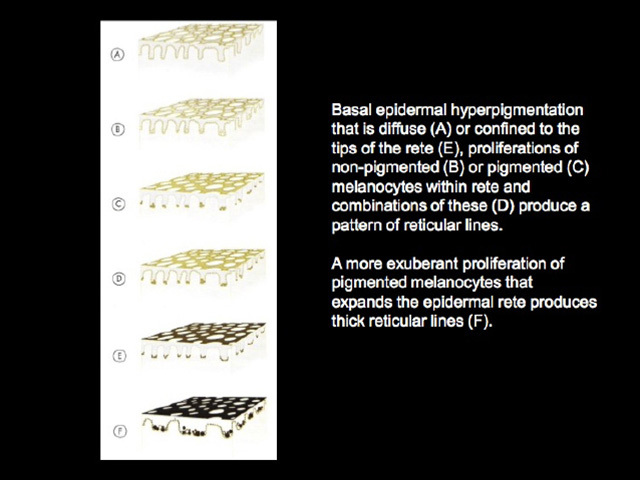
Curved lines
Curved lines are seen in dermatoscopy of pigmented keratinocytic proliferations that are either acanthotic, such as seborrhoeic keratosis, or in specific locations, such as close to mucocutaneous junctions, as with some labial and genital melanotic macules.
The dermatoscopic features of melanotic macules vary with site reflecting the differences in the anatomy of the dermoepidermal junction in normal skin (ink spot lentigo, Figures 6a, 6b) and mucocutaneous transition zones (Figures 7a and 7b). The histology is, however, reasonably similar irrespective of site (Figure 8).
Figure 6a Figure 6b 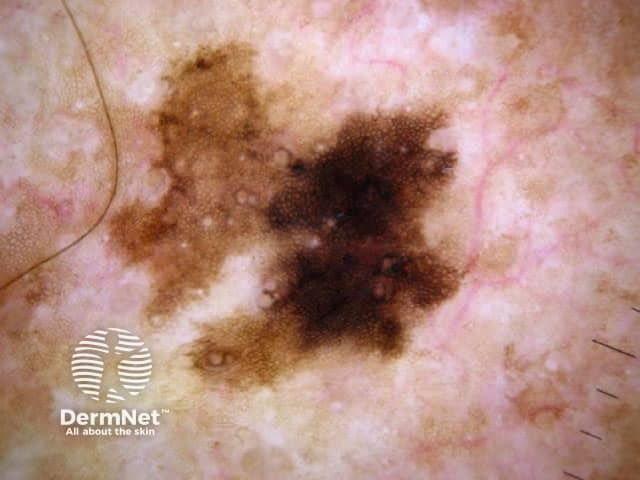
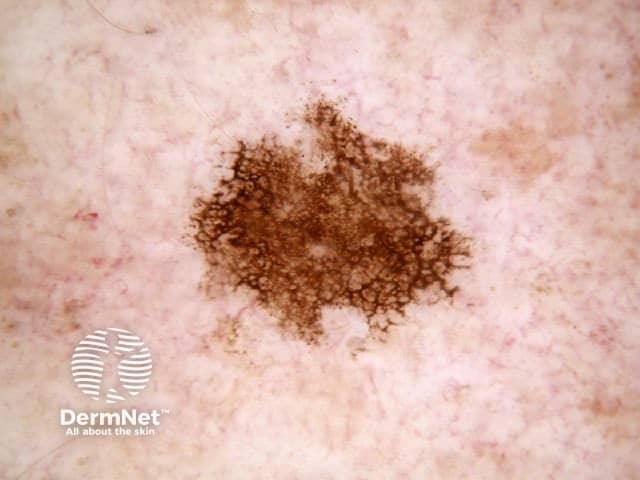
Figure 7a Figure 7b 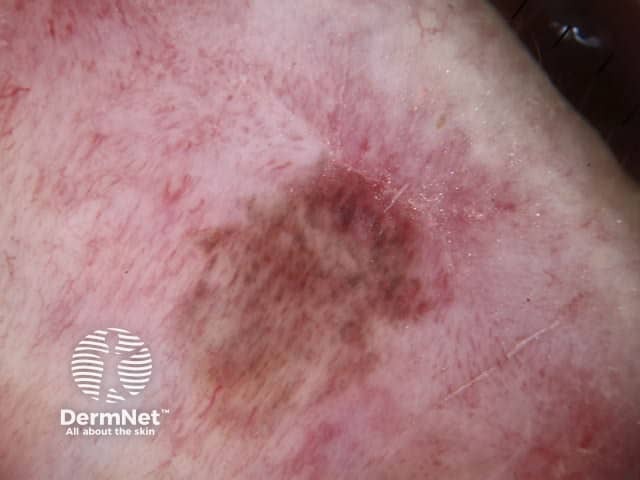
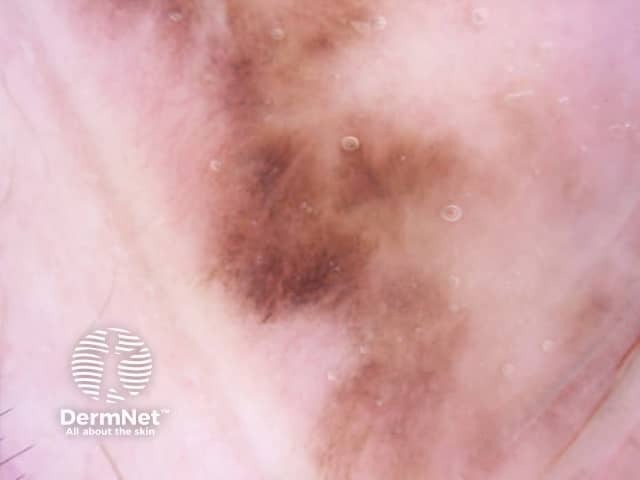
Figure 8 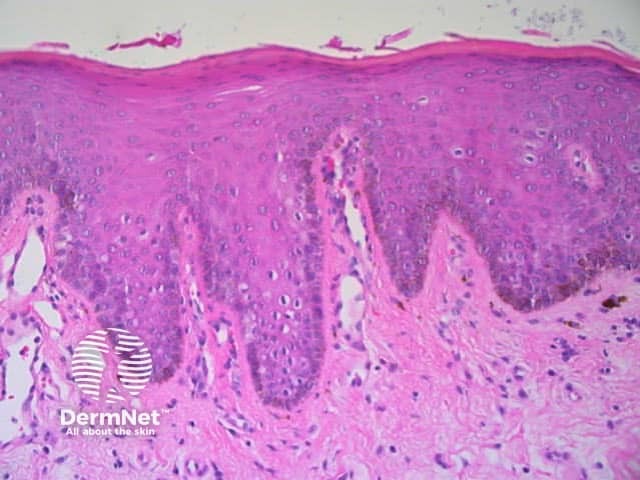
The dermatoscopic features observed in solar lentigo depend on the site of the lesion (Figures 9, 10 and 11).
Figure 9 Figure 10 Figure 11 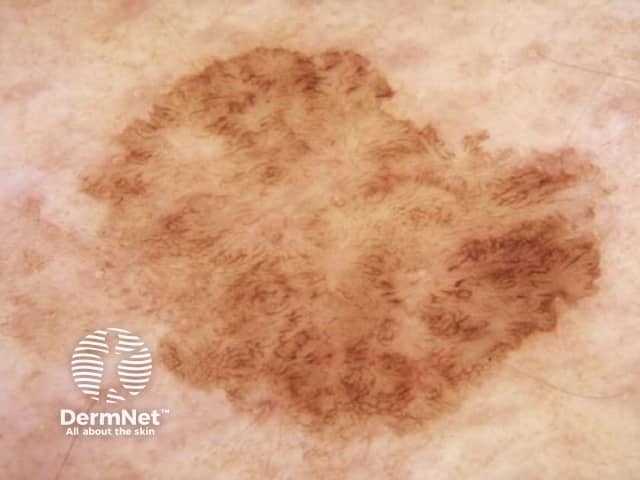
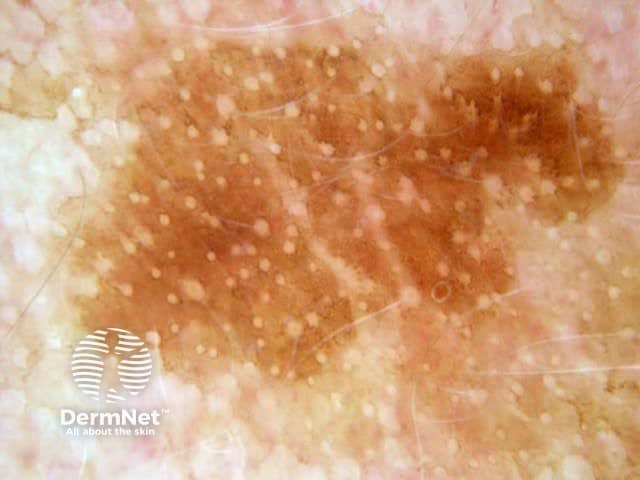
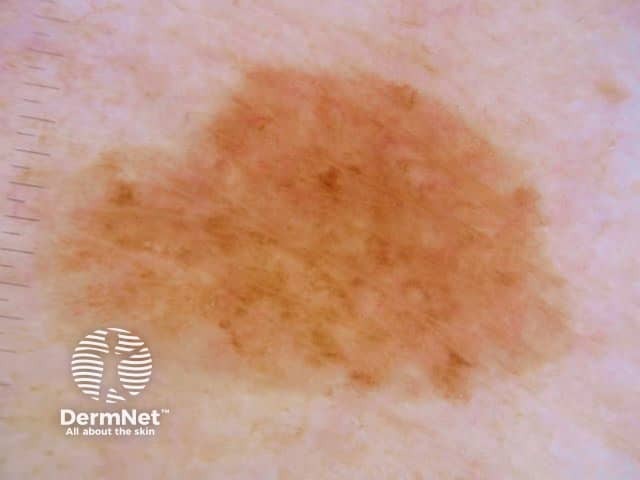
Figure 12 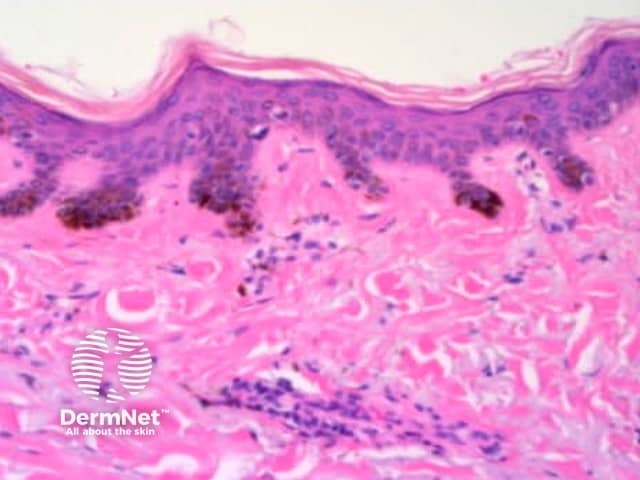
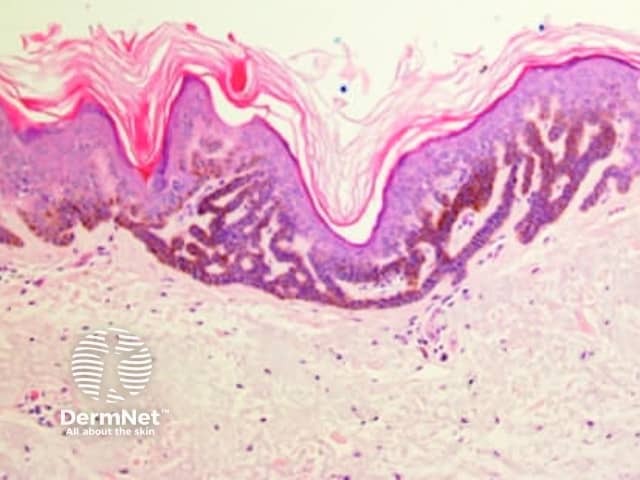
Seborrhoeic keratoses are amongst the most variable lesions on dermatoscopy, which is reflected in their protean histological appearances (Figure 15). Aside from the pseudopod pattern, any pattern or colour can be found. Flat seborrhoeic keratoses appear similar to solar lentigo on dermatoscopy. With early epidermal acanthosis thin curved lines (Figure 13) and circles become manifest, whilst with advanced acanthosis thick curved lines (Figure 14) and clods typically predominate. White clods are due to keratin under the stratum corneum. Orthokeratotic loosely laminated surface keratin appears yellow. Crypts fill with discoloured keratin and may appear various colours on dermatoscopy including brown and orange (Figure 15).
Figure 13 Figure 14 Figure 15 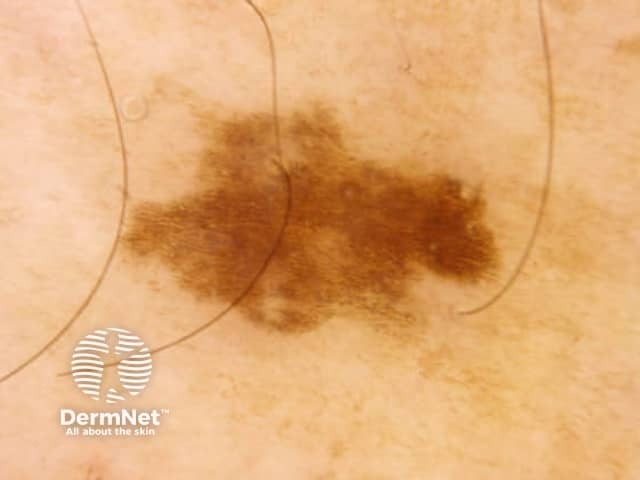
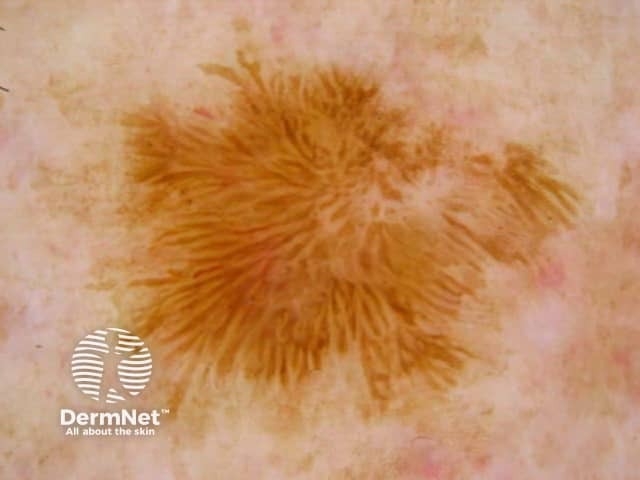
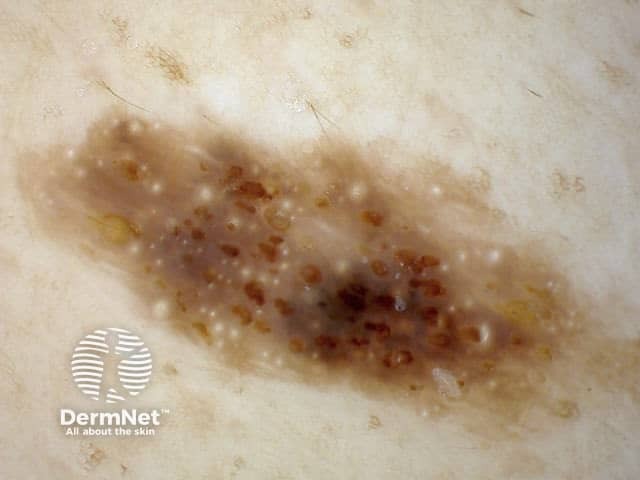
Figure 15a Figure 15b Figure 15c 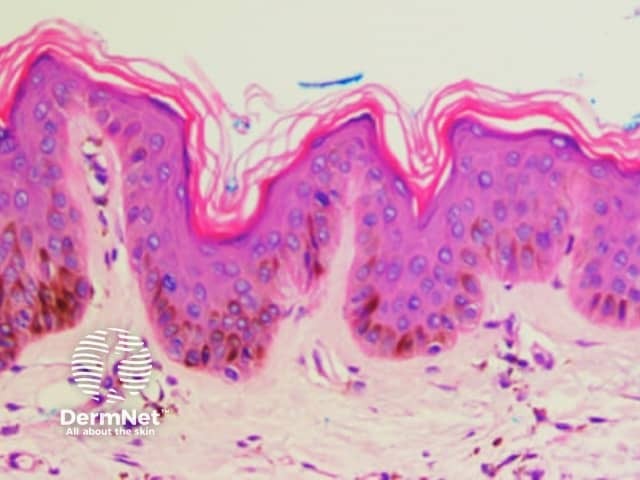
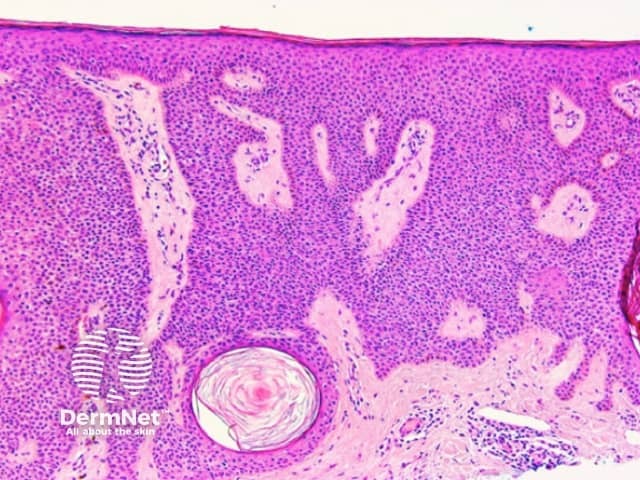
Both lentiginous and dysplastic naevi (Clark naevi) typically show a pattern of reticular lines on dermatoscopy, or less often circles (see later), that may be combined with dots, clods or structureless areas, usually in a symmetrical arrangement. Rarely only dots or clods or structureless areas are present.
Figure 16 Figure 17 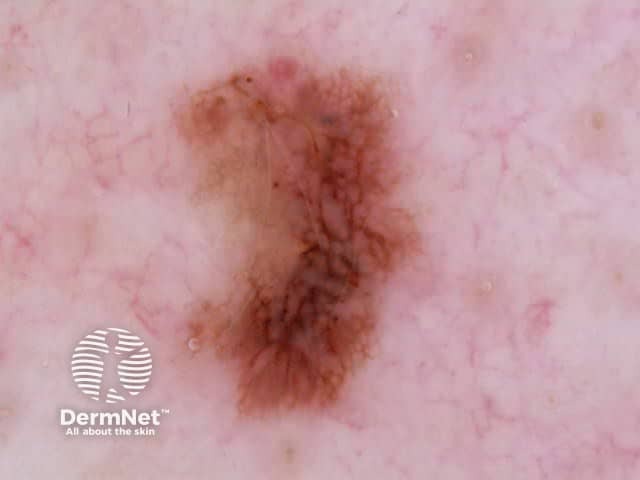
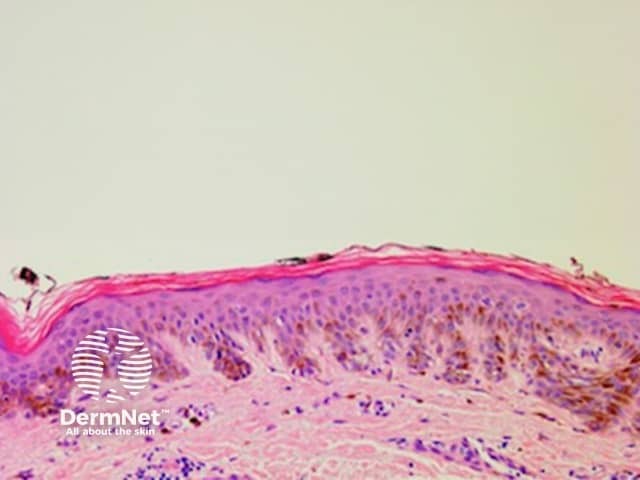
Thick lines are defined in dermatoscopy as those in which the lines are as thick, or thicker, than the spaces between them. Thick reticular or branched lines can be seen when an exuberant proliferation of pigmented melanocytes expands the epidermal rete ridges (Figure 18a,b). Thick curved lines are seen when a pigmented keratinocytic lesion produces acanthosis with thick downgrowths of pigmented epithelium as in some seborrhoeic keratoses (see previous). Thick parallel lines are discussed seperately later.
Figure 18a Figure 18b Figure 18c 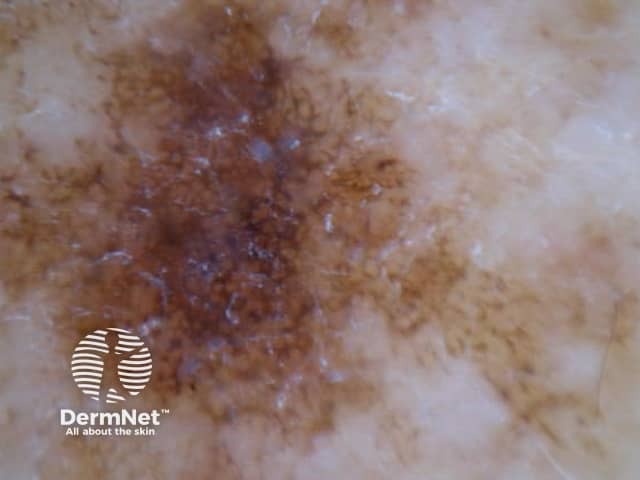
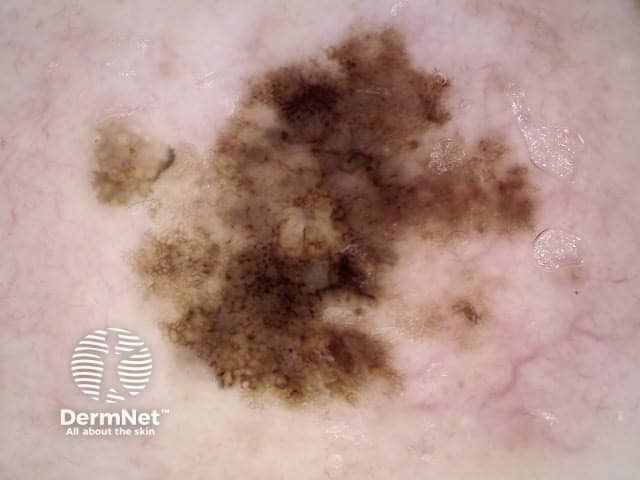
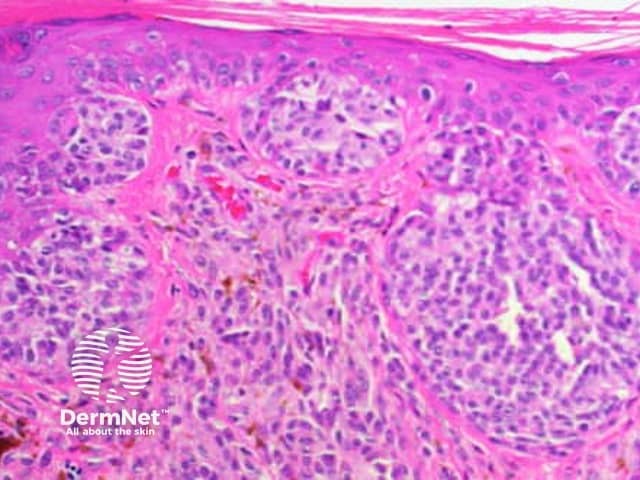
Reticular and branched lines are commonly seen in association with other dermatoscopic patterns, either symmetrically or asymmetrically. The combinations with dots and clods reflects the presence of those things that cause those patterns (eg large nests of pigmented melanocytes giving clods) in conjunction with elongate pigmented rete.
The combination of dermatoscopic structureless and reticular patterns may be due to marked pigmentation of lesional cells obscuring the pattern of lines and / or loss of the epidermal rete in part of the lesion. The former can be seen centrally in some dysplastic naevi, centrally or eccentrically in combined (true and blue) naevi and eccentrically in some melanomas. The latter may be seen with epidermal remodelling in melanoma (Figure 19a,b,c), past regression of melanocytic proliferations or due to an underlying expansile dermal lesion (Figures 20-22). The colour of the structureless zone depends on the level of the pigment within the skin: it may be white, if due to dermal fibrosis; skin coloured, if associated with epidermal remodelling in association with a proliferation of non-pigmented melanocytes; and black, brown, grey or blue, if due to heavy melanin pigmentation.
Figure 19a Figure 19b Figure 19c 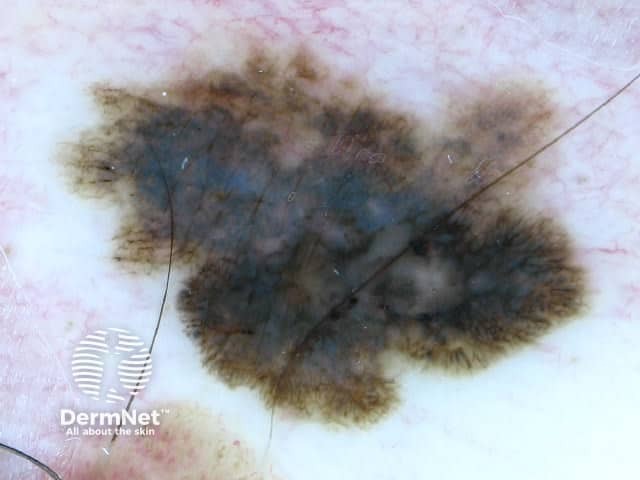

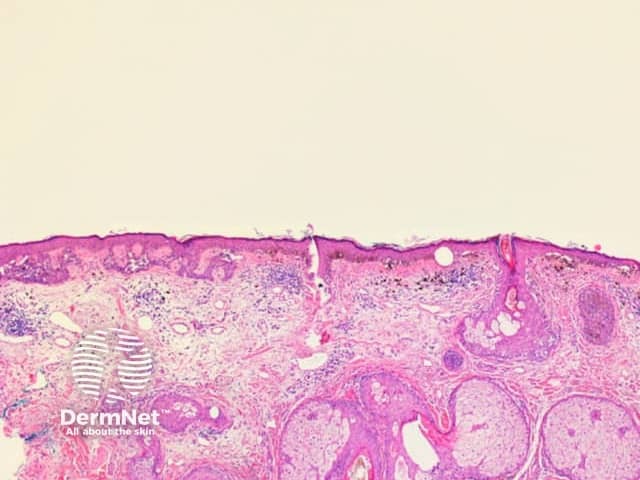
Figure 20 Figure 21 Figure 22 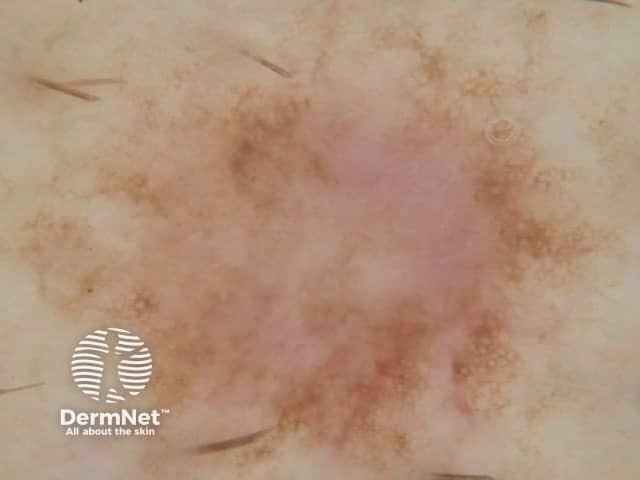
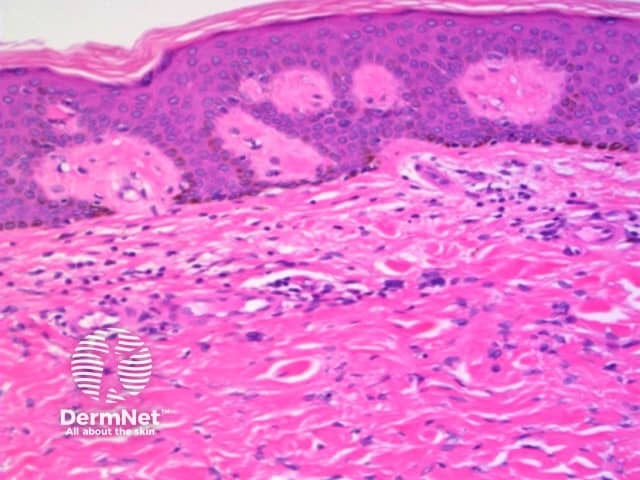
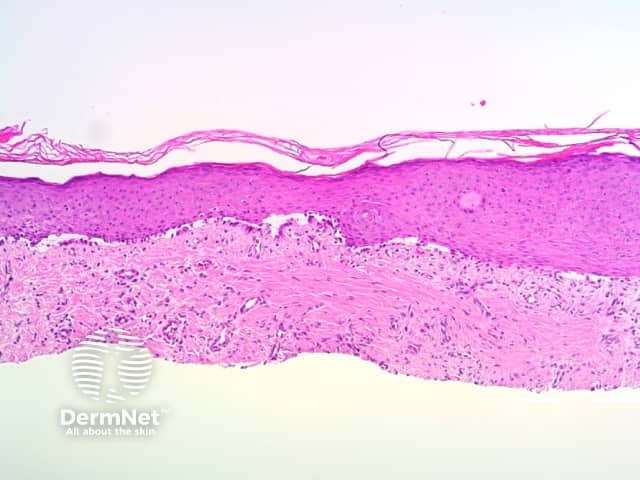
Parallel lines are seen on dermatoscopy on acral skin and reflect the unique anatomy of the dermoepidermal junction in palmar and plantar skin. Surface ridges (on which eccrine ducts open to the surface) overlie the crista profunda intermedia whilst surface furrows overlie the crista profunda limitans (Figure 23). The different sites of predilection for proliferation of acral naevus cells and acral melanoma cells result in parallel furrow and parallel ridge patterns respectively (Figures 24, 25, 27 and 28). Exceptions do, however, occur (Figure 26a and 26b). Acral melanoma may also show structureless pigmentation reflecting proliferation of pigmented melanocytes unrelated to the normal epidermal architecture.
Acral volar skin. Acral naevus
Figure 23 Figure 24 Figure 25 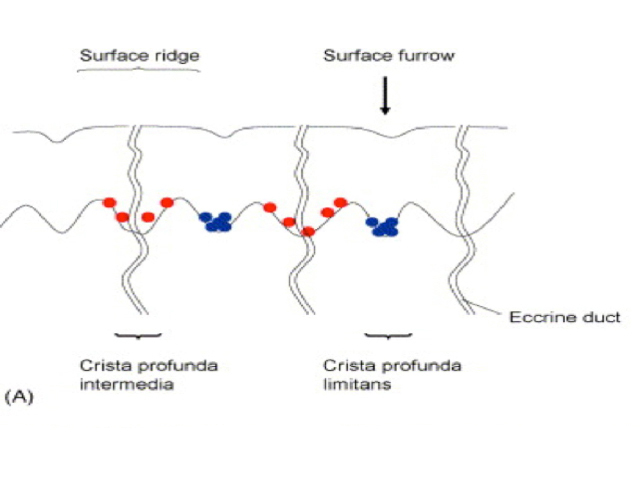

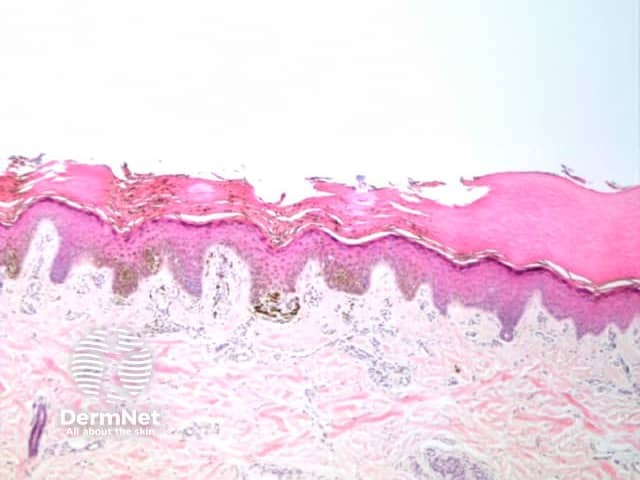
Figure 26a Figure 26b 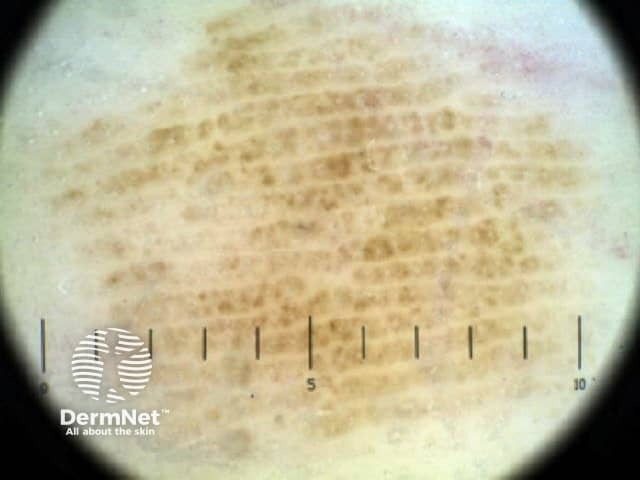

Figure 27 Figure 28 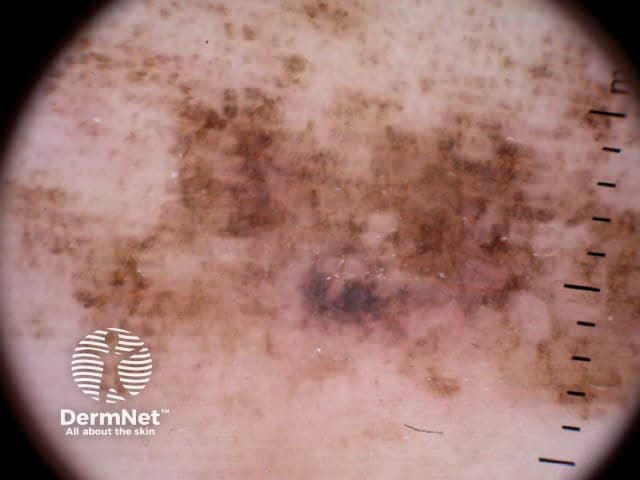
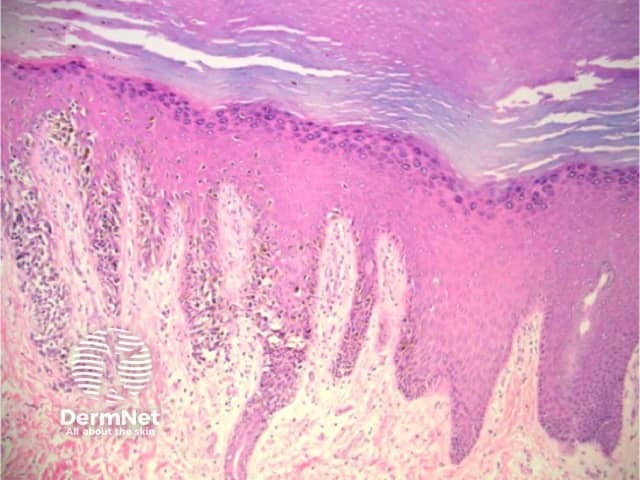
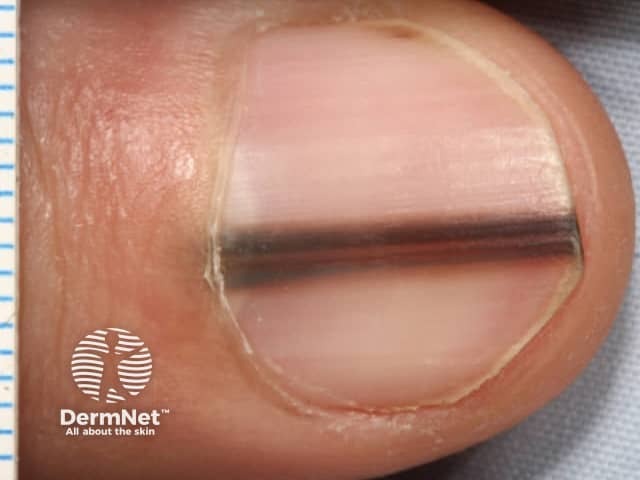
Pigmented nail matrix lesions, including nail matrix melanoma, typically present with longitudinal melanonychia: a pigmented stripe running from the cuticle to the free edge of the nail. The primary lesion arises in the germinal matrix deep to the proximal nail fold and proximal nail plate and melanin is transferred to the developing nail plate and growth of the lesion may manifest as increasing width of the stripe. Extension of the primary lesion to the proximal nail fold (Hutchinson sign) is a clinical clue, albeit relatively late, to the diagnosis of nail matrix melanoma (Figures 29 and 30).
Examination of the pigmented stripe can reveal the hallmark clues of pigmented nail-matrix melanoma: longitudinal melanonychia with lines parallel chaotic; lines parallel varying in width, interval and colour.
Figure 29 Figure 30 Figure 31 Figure 32 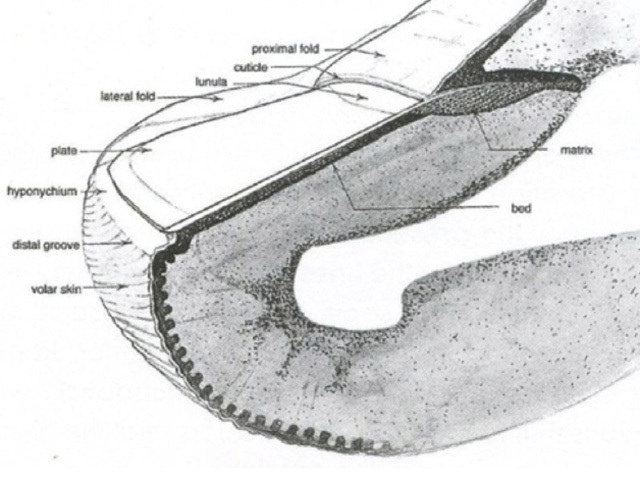


It is essential that nail matrix biopsy be performed competently so that adequate tissue is obtained to facilitate histological diagnosis. One method is the ‘fenestration’ technique (4) but the authors now prefer the ‘pop the bonnet’ technique as this allows direct visualisation of the entire matrix including dermatoscopy of nail bed and nail plate (figure 33a, b).
Figure 33a Figure 33b 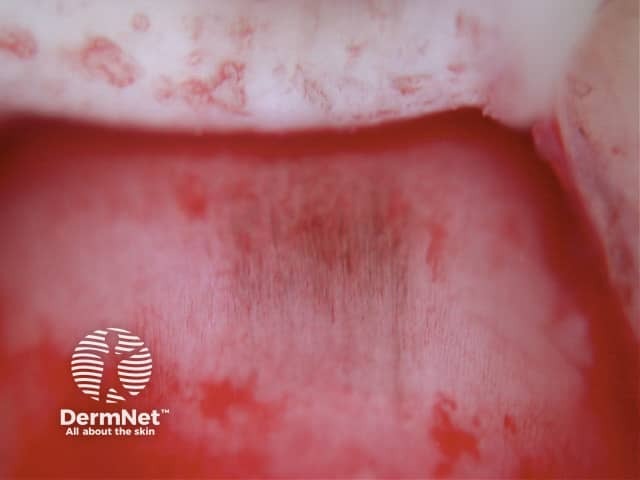

The biopsy should ideally include matrix from the proximal edge of pigment on the nail matrix to the distal edge of the matrix (located at the lunula). It is essential that nail matrix tissue is examined by a dermatopathologist cognisant of the challenges this can present. A recent publication asserts that immunoperoxidase stains may be necessary to safely exclude malignancy (5).
Dots are small enough to have no discernable shape at the magnification provided by a dermatoscope. They represent melanin, or occasionally haemosiderin, with the colour depending on the level of the pigment within the skin in the case of melanin. Dots may be black (superficial epidermal melanin), brown (basal epidermal melanin or superficial dermal haemosiderin), grey (papillary dermal melanin) or blue (reticular dermal melanin). They are usually present in association with another pattern. Brown or grey dots are occasionally found without another pattern (Figures 34-36). Red dots, representing a vascular pattern, are discussed later.
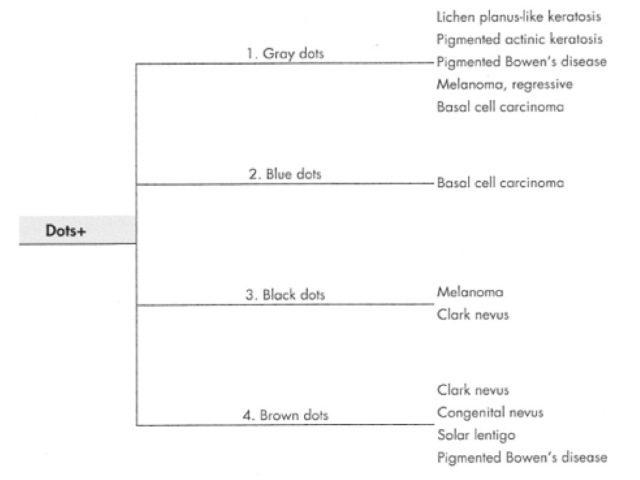
Black dots result either from pagetoid pigmented melanocytes or free pigment within the stratum corneum. Brown dots reflect either small nests of melanocytes in the basal epidermis, focal pigmented keratinocytic proliferation, as seen in some forearm solar lentigines, or superficial dermal haemosiderin deposition. Grey dots are due to melanin pigment within the papillary dermis, either free, in small nests of melanocytes or, more commonly, in melanophages. Blue dots reprresent deeper dermal melanin. The differential diagnosis of dots on dermatoscopy is shown in Figure 34.
Figure 34a Figure 34b Figure 35 Figure 36 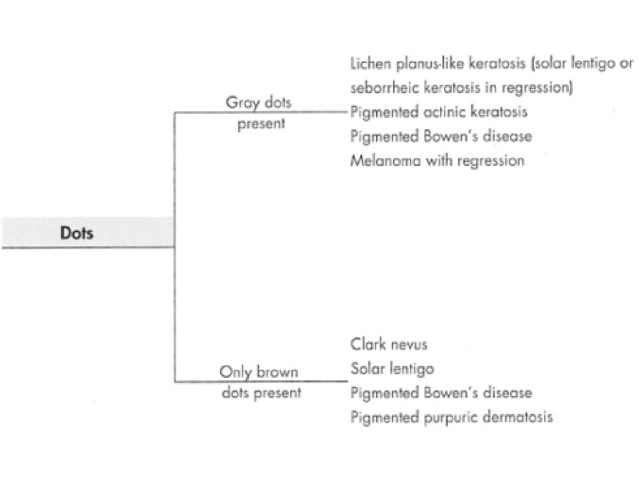

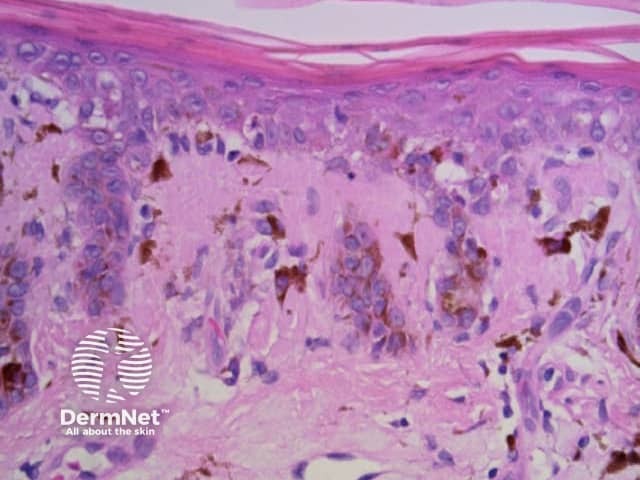
Clods are solid rounded structures of sufficient size to show an appreciable variability in size. Their colour depends on the nature and, in the case of melanin, depth of the substance they comprise (Figures 37-50). Other terms have been used for clods in specific settings such as blue-grey ovoid nests for grey and / or blue clods seen in basal cell carcinoma.
Figure 37a Figure 37b 
Figure 38 Figure 39 Figure 40 Figure 41 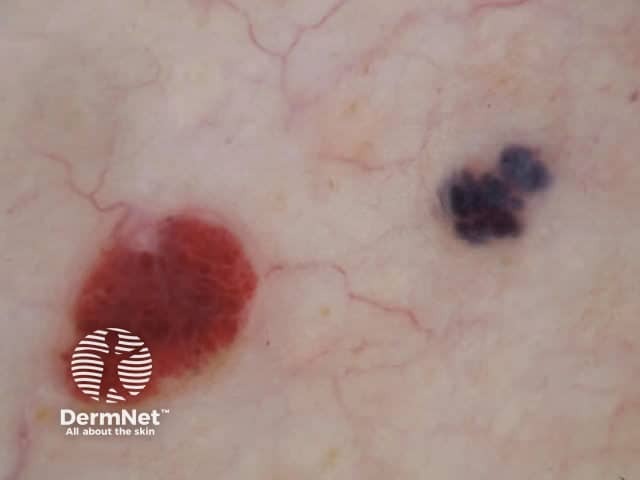
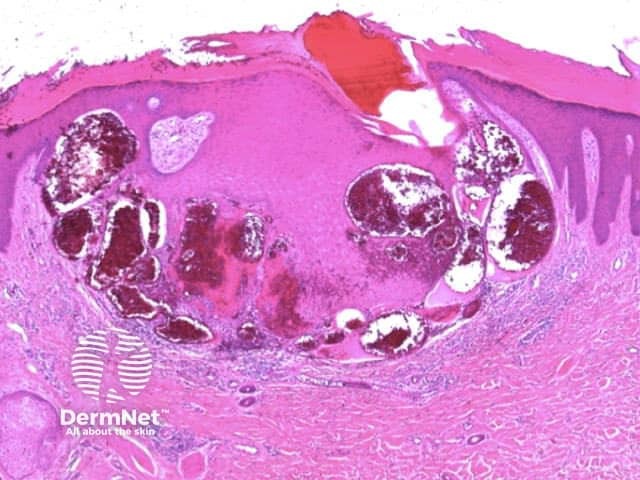
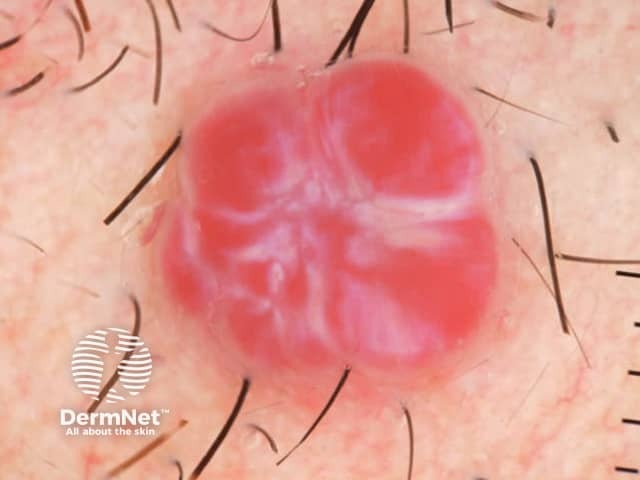
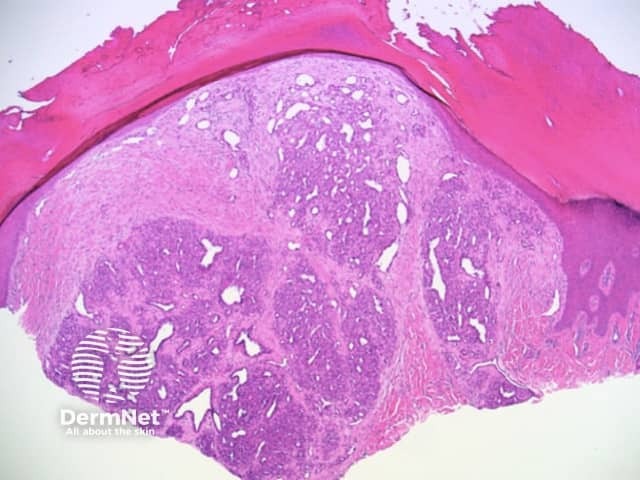
Figure 42 Figure 43 Figure 44 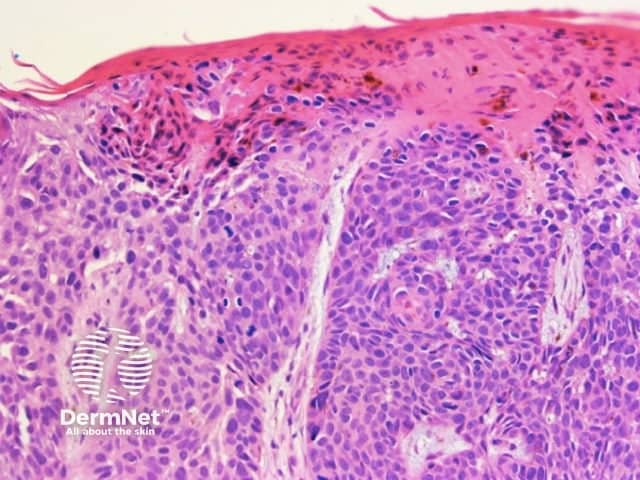
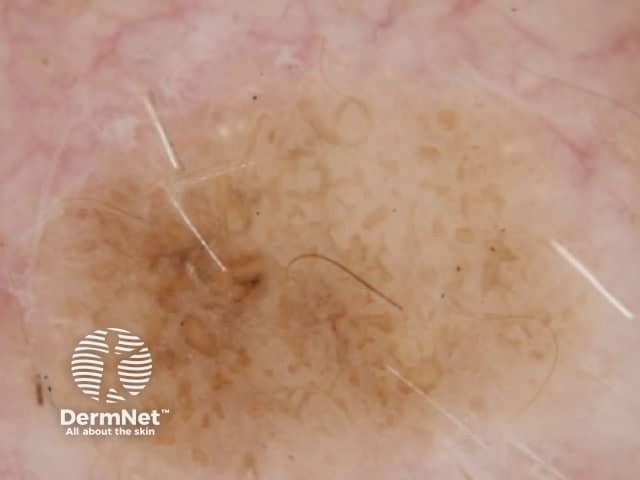
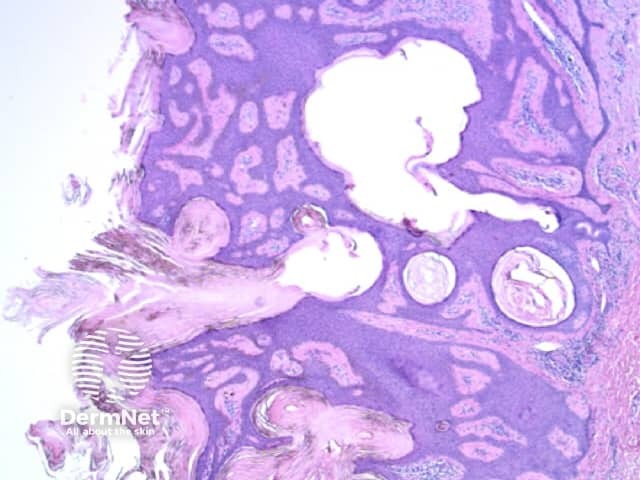
Figure 45a Figure 45b Figure 46a Figure 46b 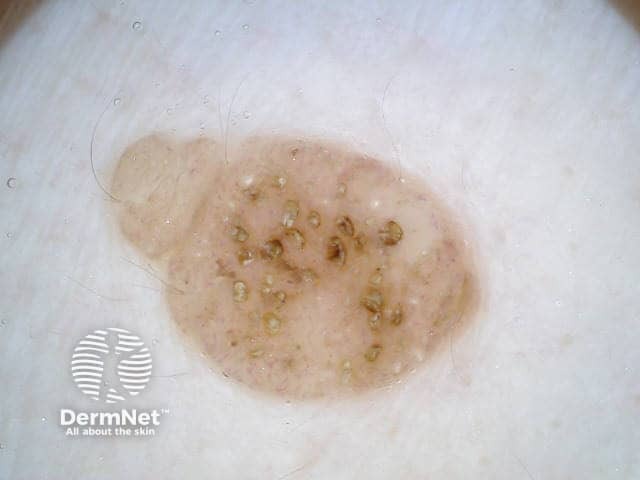
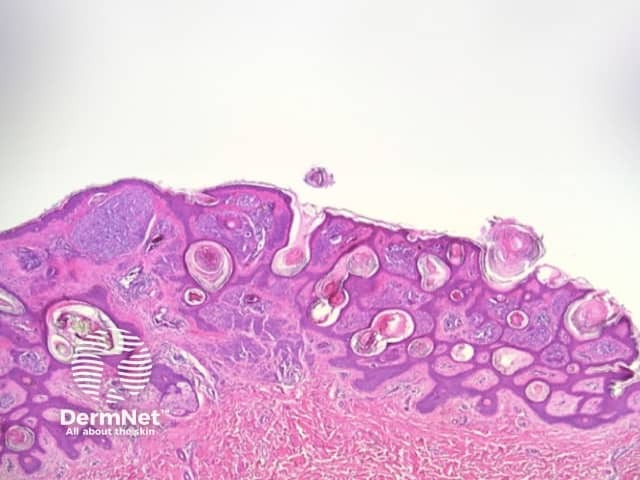

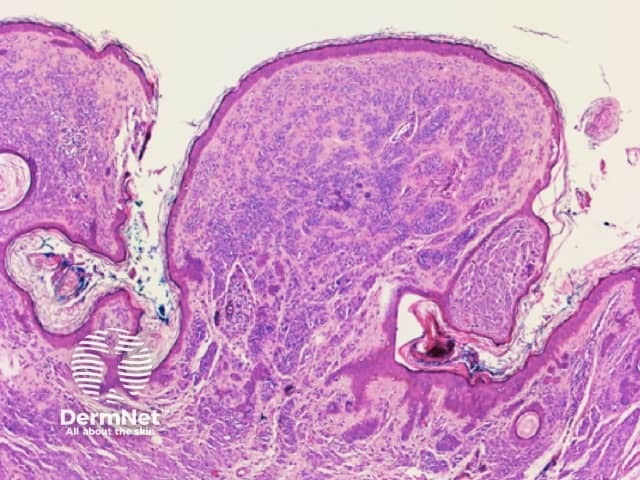
Figure 47 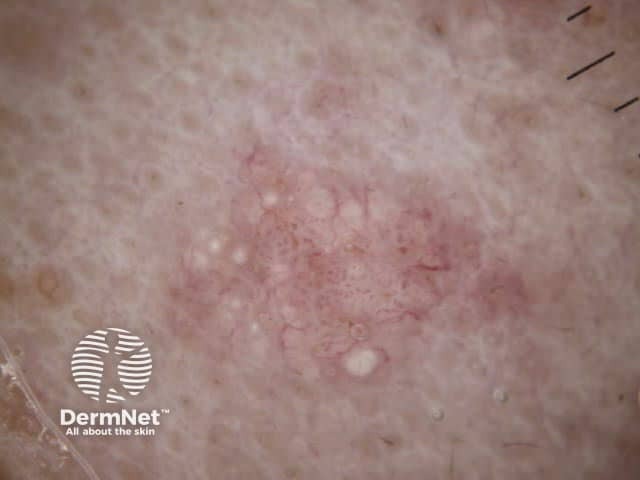
Figure 48 
Figure 48. Diagram. Brown, grey and blue clods are due to nests of melanin-containing cells at different levels in the skin. From Kittler et al (1).
Figure 49a Figure 49b Figure 49c Figure 49d 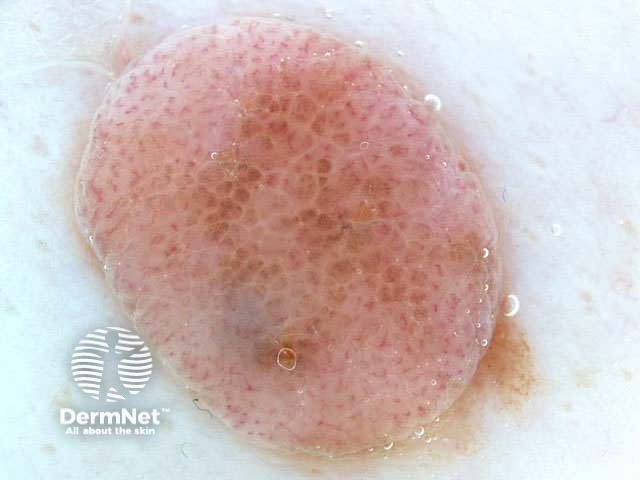
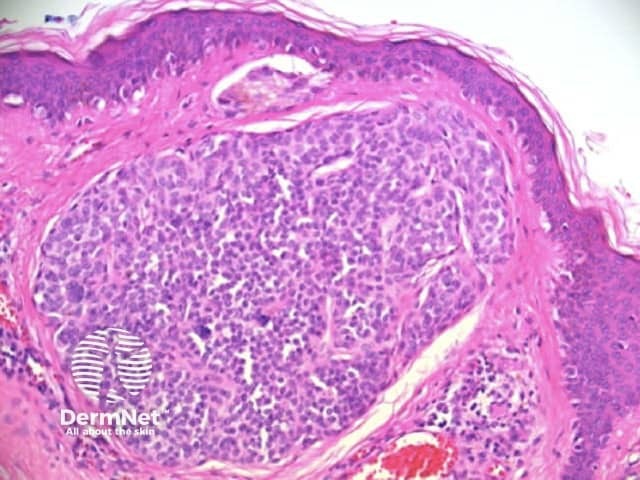
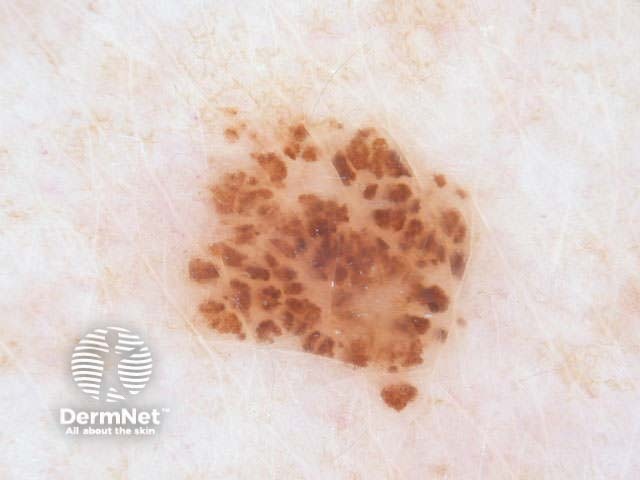
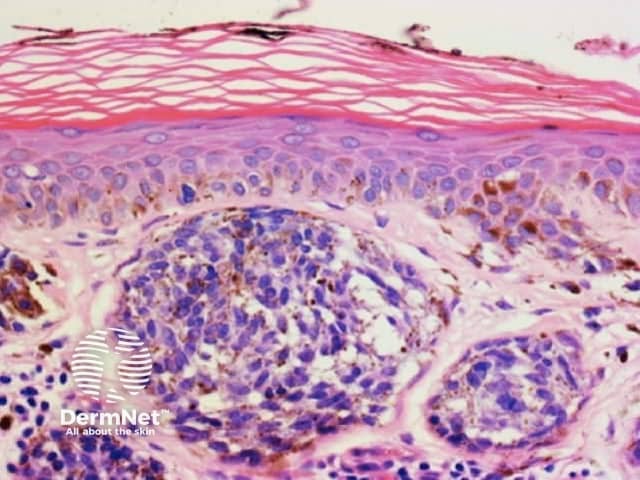
Figure 50a Figure 50b 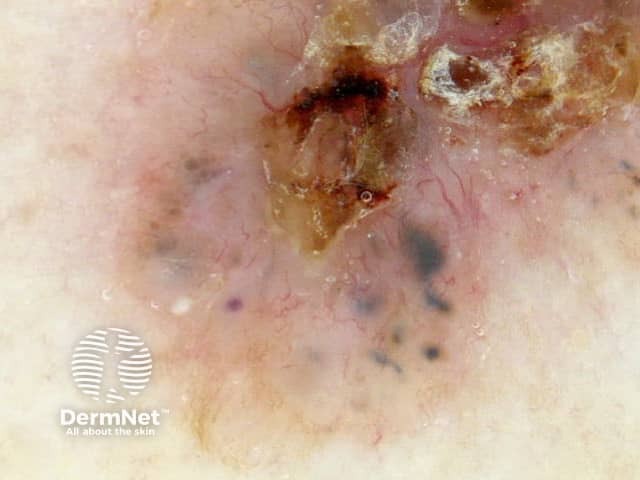
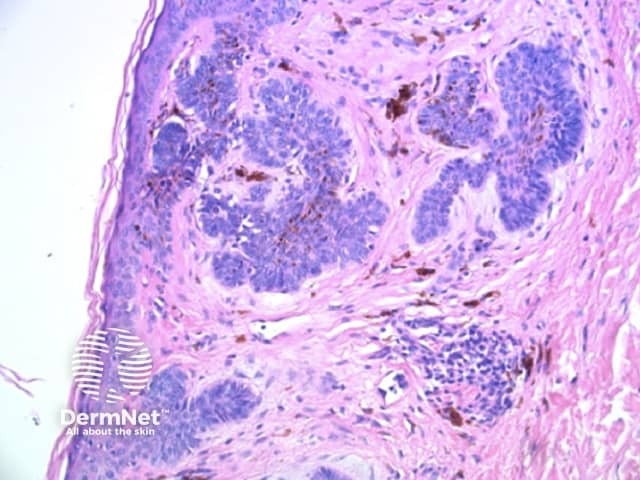
Circles can be seen on non-facial skin where they arise by expansion of rete so that the relatively less pigmented base becomes discernible (Figure 51).
Circles are much more commonly seen on facial skin, reflecting epidermis with flattened rete (Figure 52) with regularly placed pigmented follicular infundibula (Figures 53-59). Differentiation between solar lentigo, pigmented actinic keratosis and melanoma-in-situ on facial skin can be difficult or, in some cases, impossible by dermatoscopy.
Figure 51 Figure 53 Figure 52 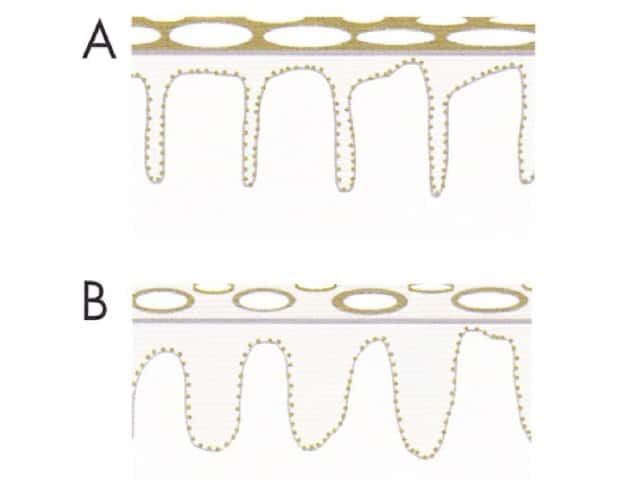
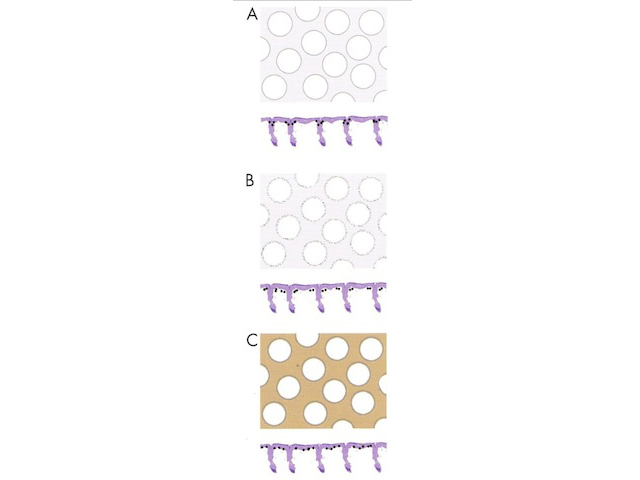
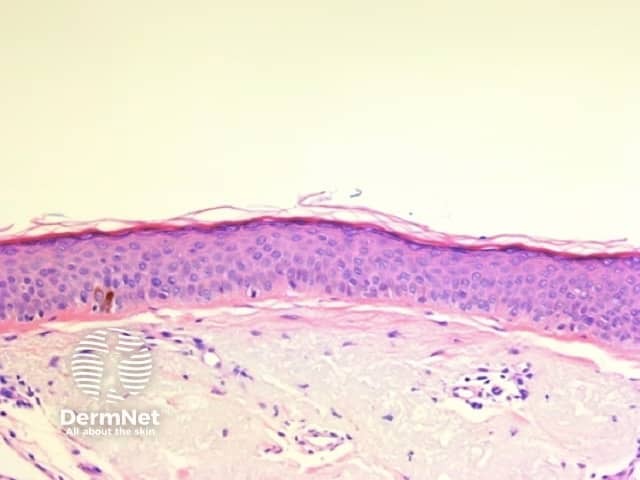
Figure 54 Figure 55 Figure 56 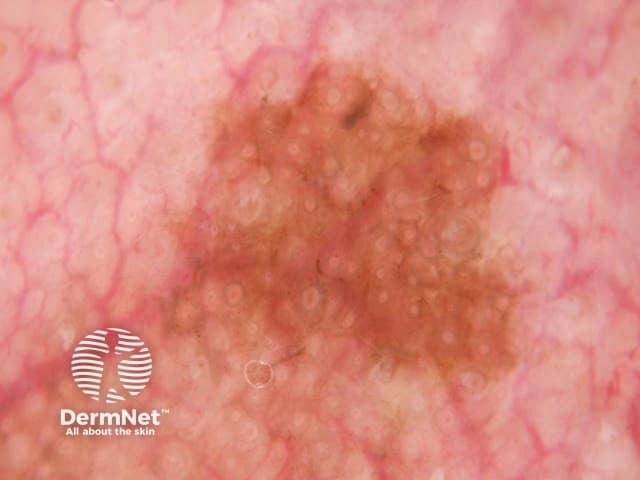
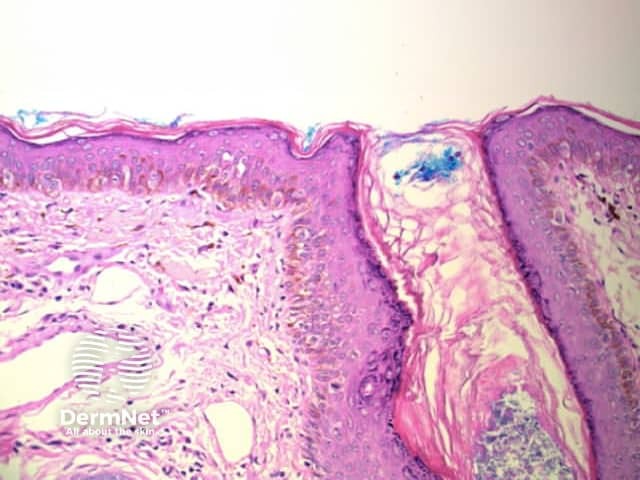
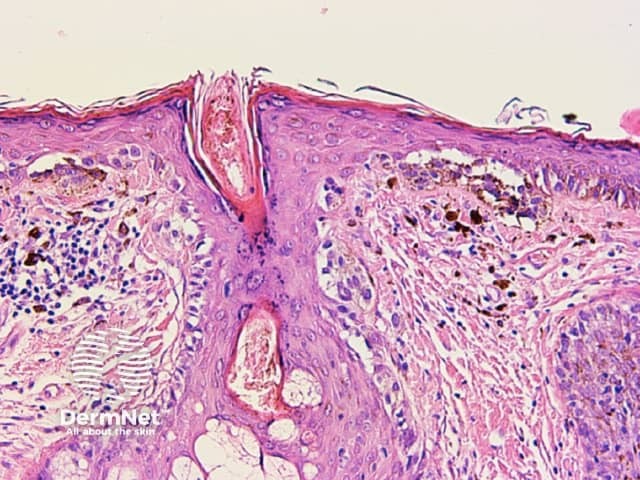
Figure 57 Figure 58 Figure 59 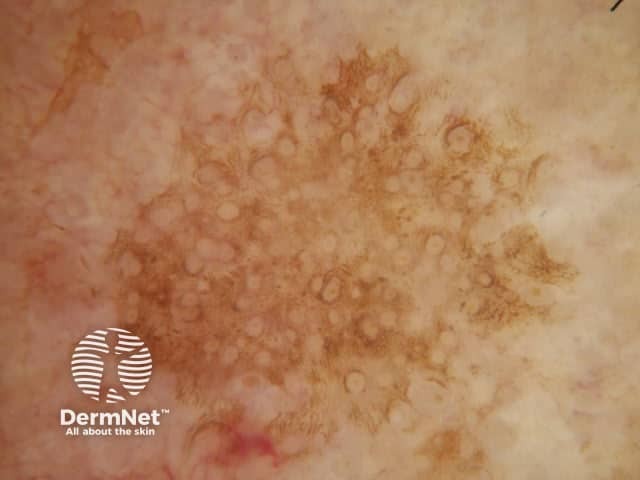
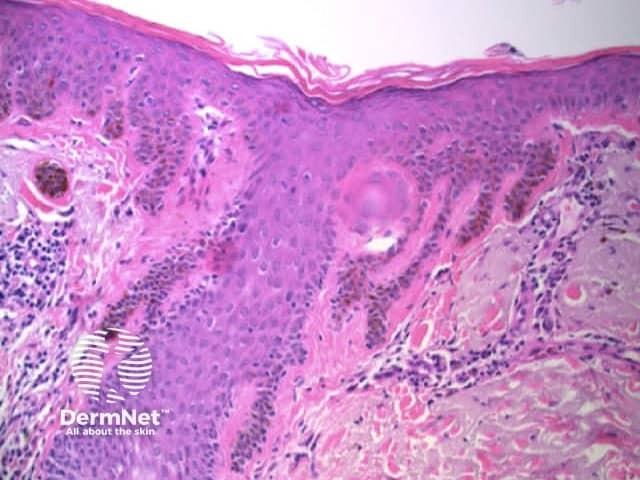
Pseudopods are structures that resemble radial lines but that have a bulbous outer end. They only occur in combination with other patterns with the typical arrangement being pseudopods at the periphery and the other pattern centrally. The second pattern is most often structureless, with clods next in frequency, and reticular lines least common.
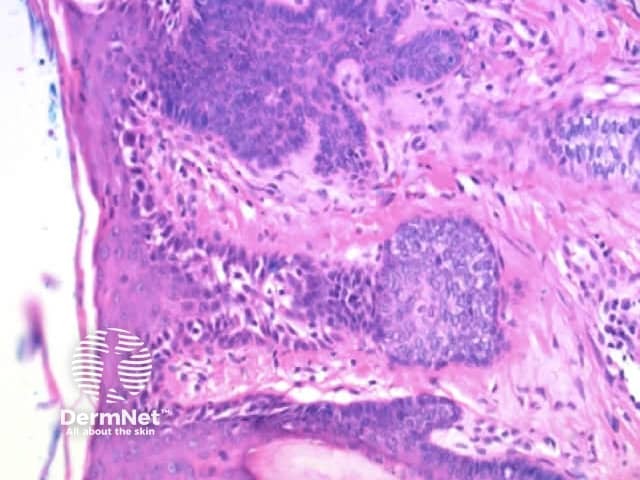
Regularly distributed circumferential pseudopods suggests a pigmented spindle cell (Reed) naevus whereas segmental pseudopods create asymmetry and are a clue to melanoma. In both cases, the histologic correlate is large nests of pigmented melanocytes at the periphery of the lesion (Figure 60).
Figure 60a Figure 60b 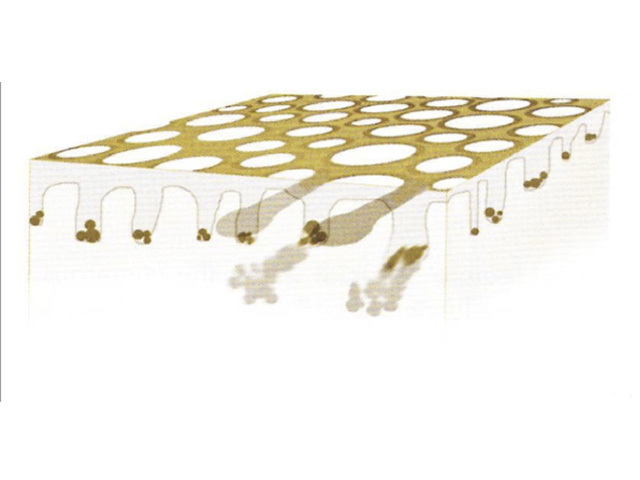
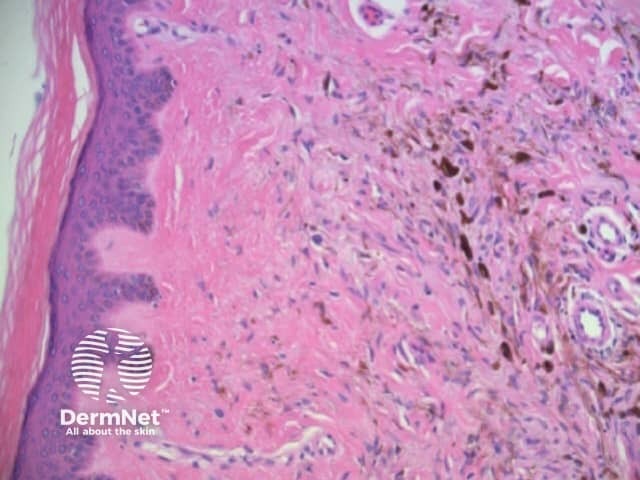
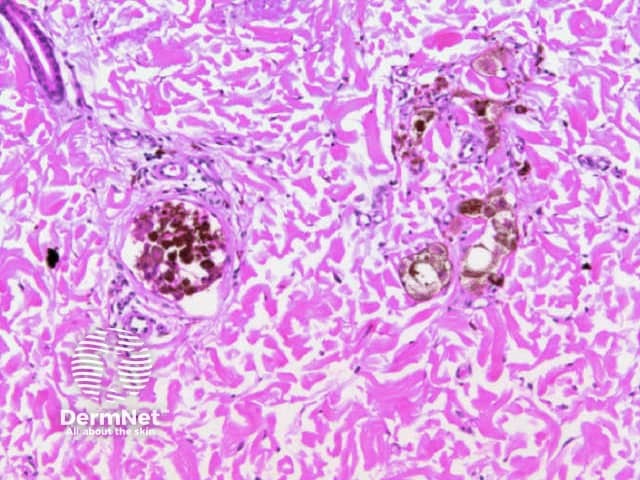
Structureless areas (Figures 61-64) are seen when:
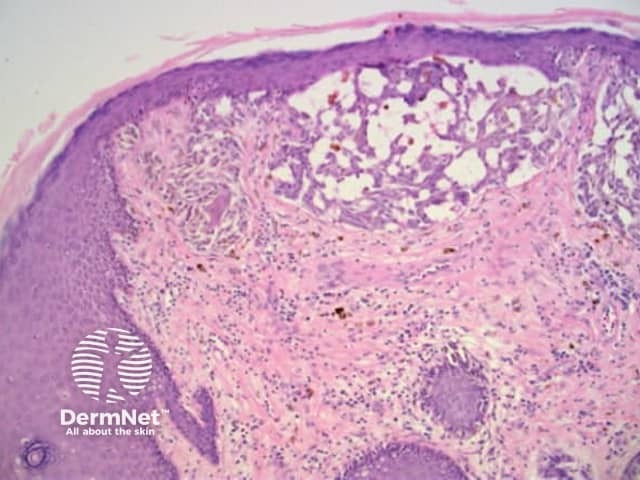
Figure 61 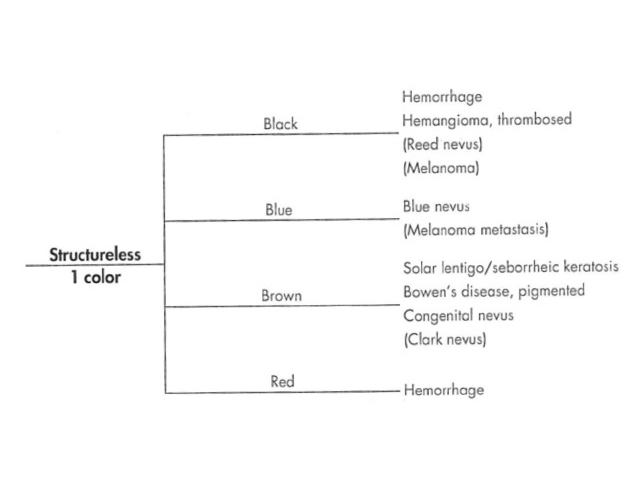
Figure 62 Figure 63 Figure 64 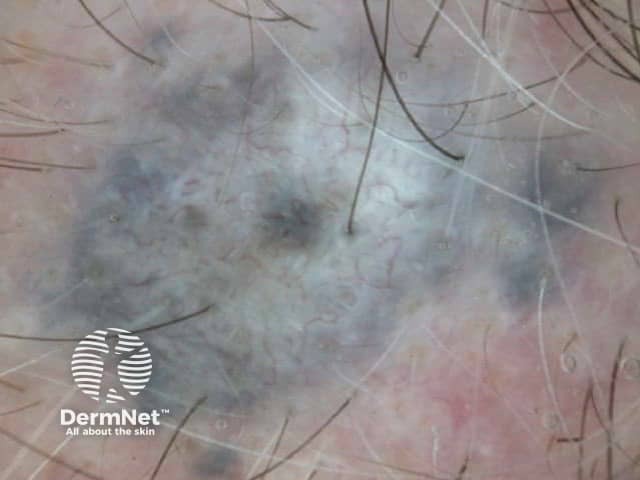
Using the algorithmic method for dermatoscopic diagnosis of pigmented lesions involves the analysis of pattern and colour to arrive at a differential diagnosis. Clues are then used to favour one diagnosis over another. A clue may be a pattern (eg eccentric structureless zone as a clue to melanoma), an arrangement of pattern / colour (eg central white structureless and peripheral reticular lines or circles in dermatofibroma), a feature too localized to form a pattern (eg blue clods as a clue to pigmented basal cell carcinoma), the absence of a feature, or a particular vascular pattern (eg branched vessels as a clue to basal cell carcinoma).
The correlation of many of these clues with histology has been covered in the earlier discussion. The significance of vascular patterns will be addressed in the assessment of non-pigmented lesions. It is important to note, however, that other than in the context of primary vascular lesions presenting as red-purple clods, as discussed previously, there is a poor correlation between histology and vascular patterns on dermatoscopy as these patterns are best appreciated when viewed in three dimensions and from above.
Pigment, when present, is typically used to generate a dermatoscopic differential diagnosis using the algorithms mentioned previously and covered in the DermNet section on dermatoscopic diagnosis. When pigment is not present, other features must be used for diagnosis. The key features used include the presence or absence of non-traumatic ulceration, the recently described “white clues” and vascular patterns (Figures 65-80).
Figure 65 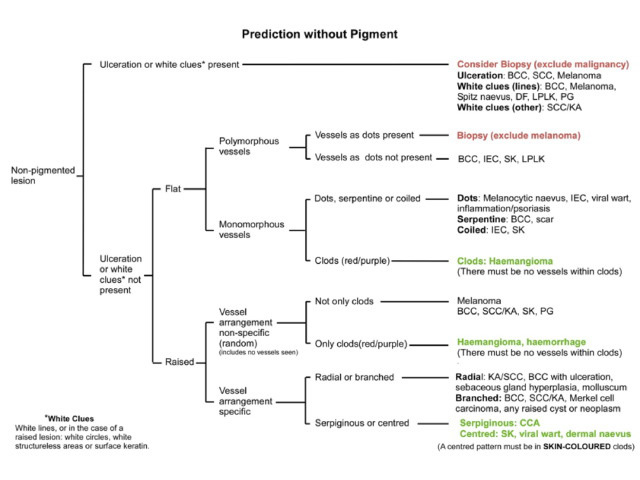
These recently described clues are of great importance in the diagnosis of certain non-pigmented lesions. The term “white clues” refers to white lines or, in a raised lesion (defined as a visibly or palpably raised lesion or a lesion with looped vessels on dermatoscopy), keratin, white circles or white structureless zones. See Figure 72[6].
Abstract of publication describing white areas in SCC
Figure 66 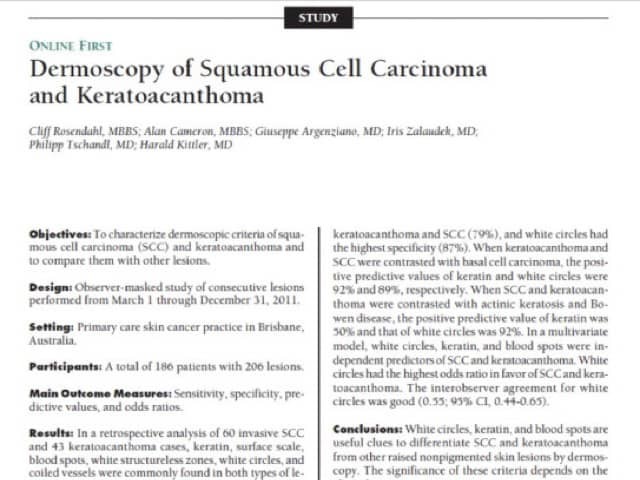
White areas in SCC
Figure 67 Figure 68a Figure 68b Figure 68c Figure 69 Figure 70a Figure 70b Figure 70c Figure 70d 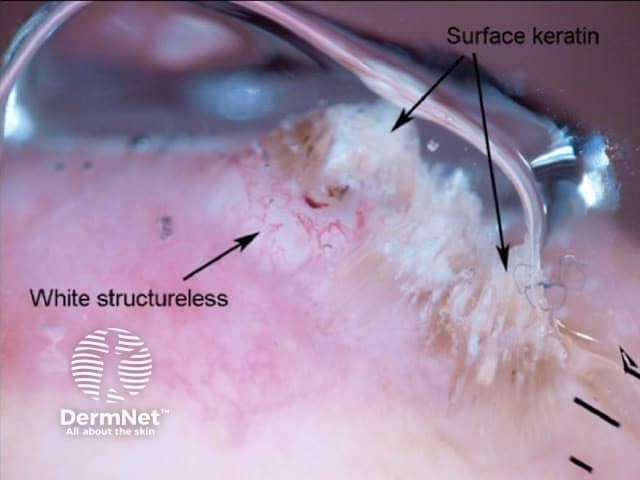
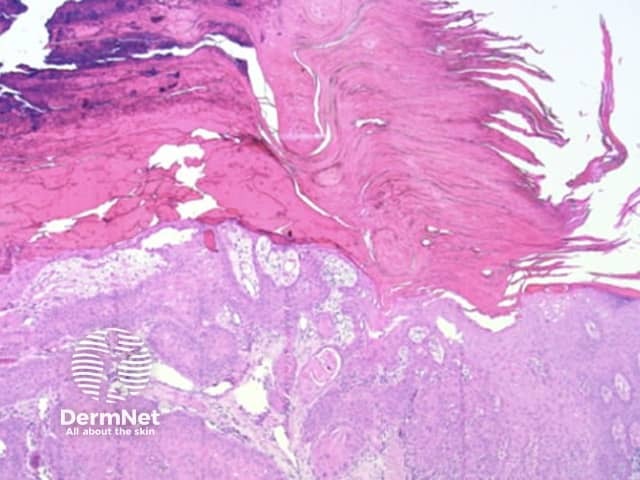
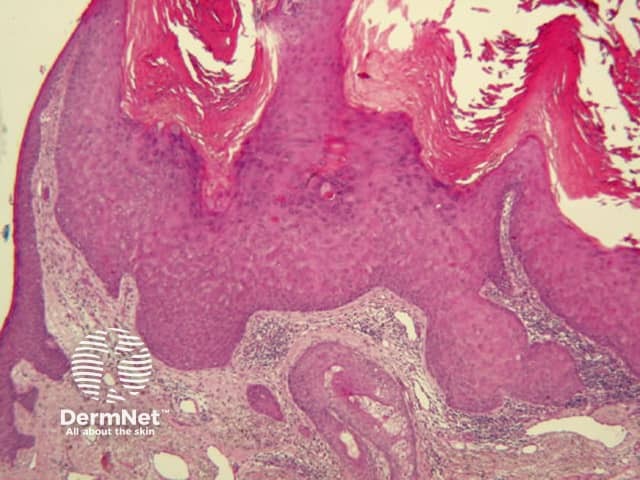
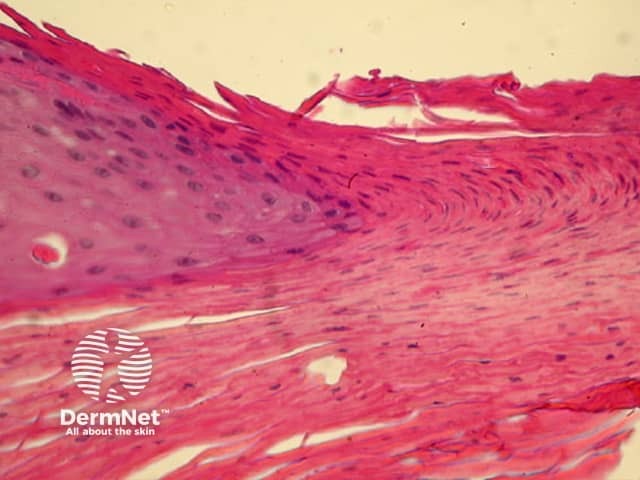
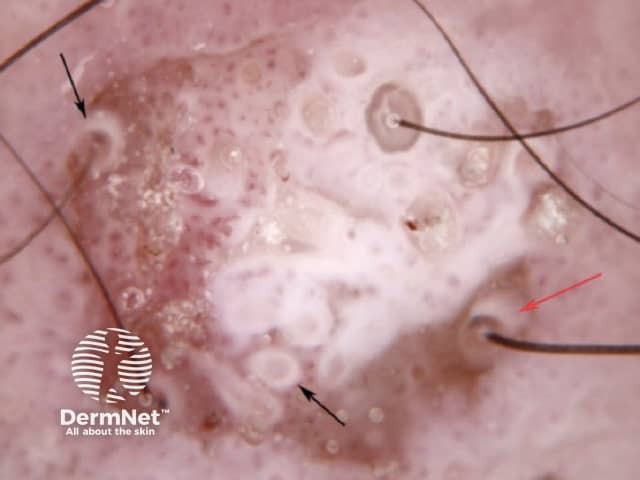
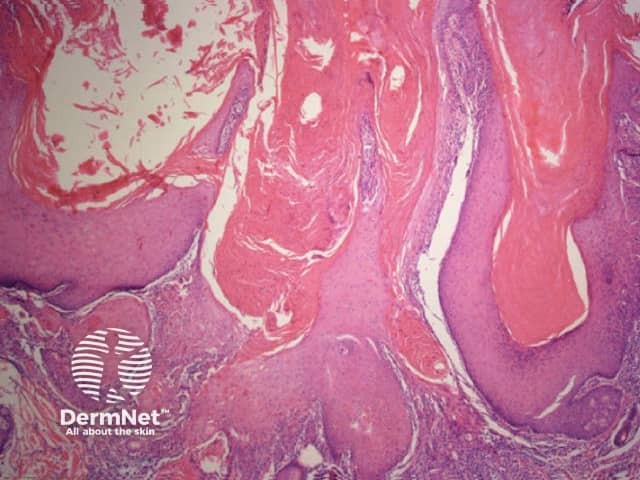
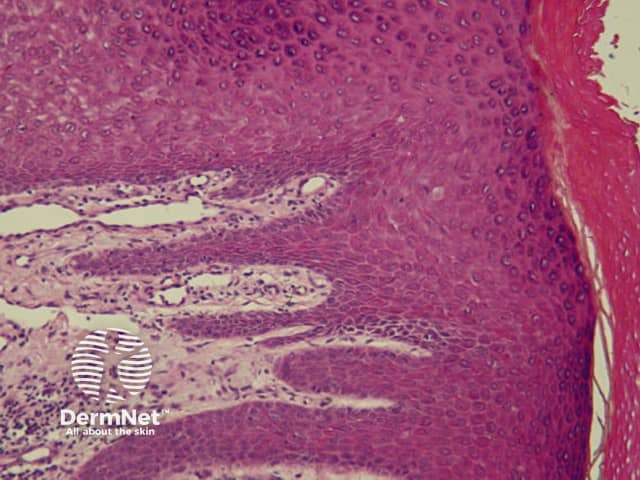
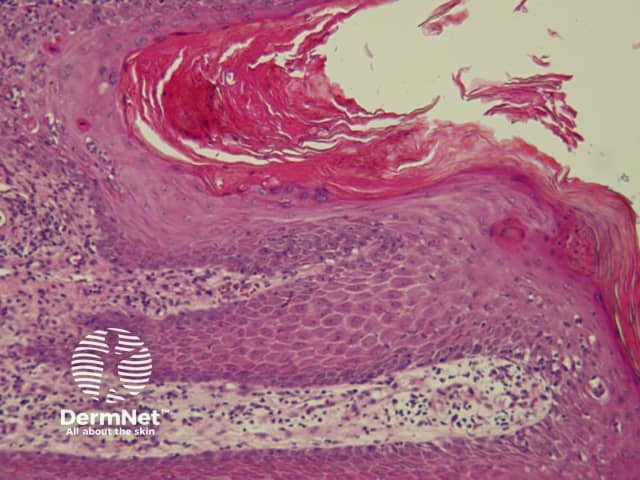
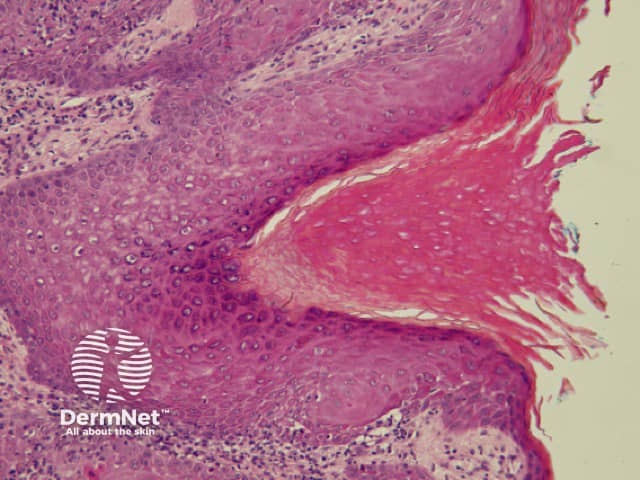
The pattern of blood vessels can be a clue to the diagnosis of pigmented lesions and is a key component of the assessment of non-pigmented lesions by dermatoscopy. In general there is a relatively poor correlation between the dermatoscopic vascular patterns and the histology of particular lesions. This is of little importance as non-pigmented lesions are obviously visible following staining of tissue sections for histology precluding the need to assess secondary features.
Figure 71 Figure 72 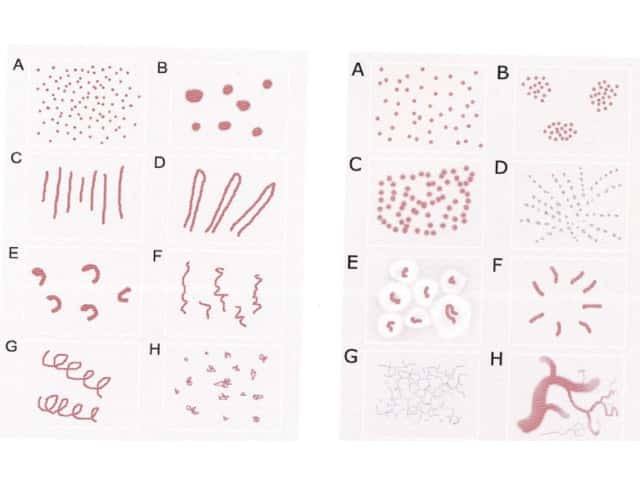
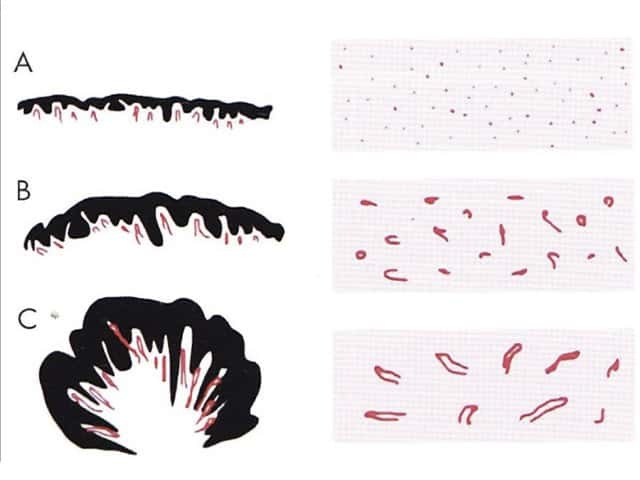
Figure 73 Figure 74 Figure 75 Figure 76 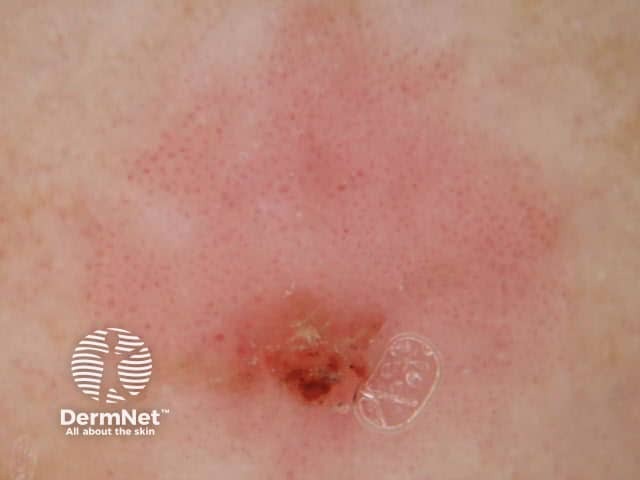
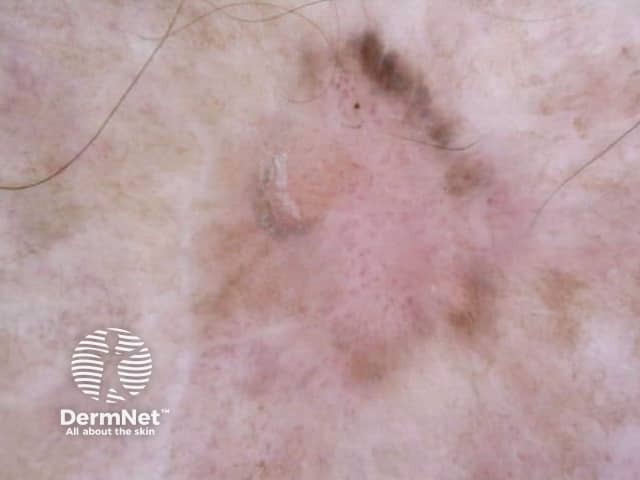
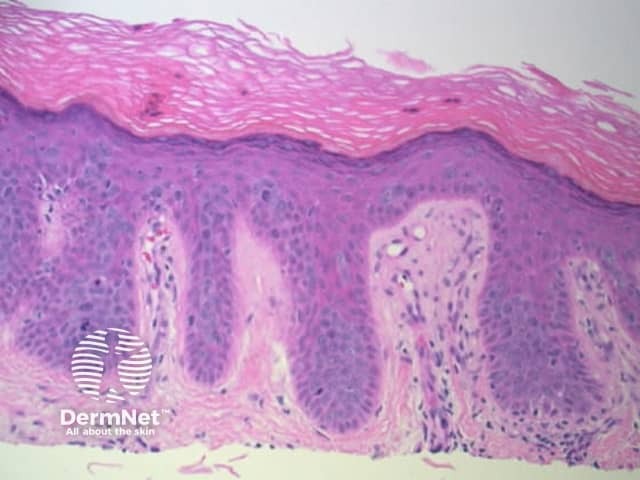
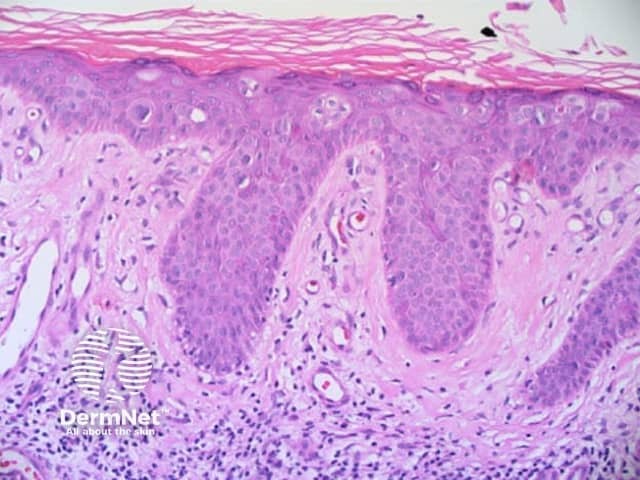
Figure 77 Figure 78 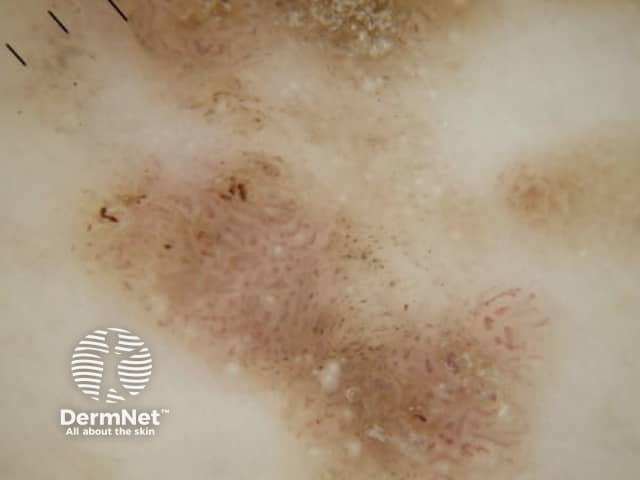
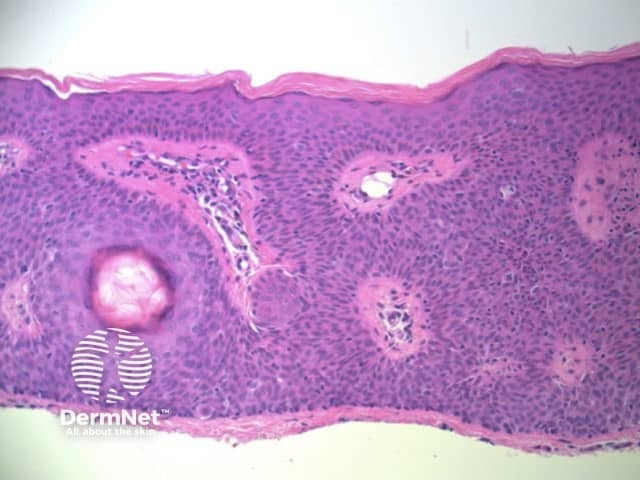
Figure 79 Figure 80 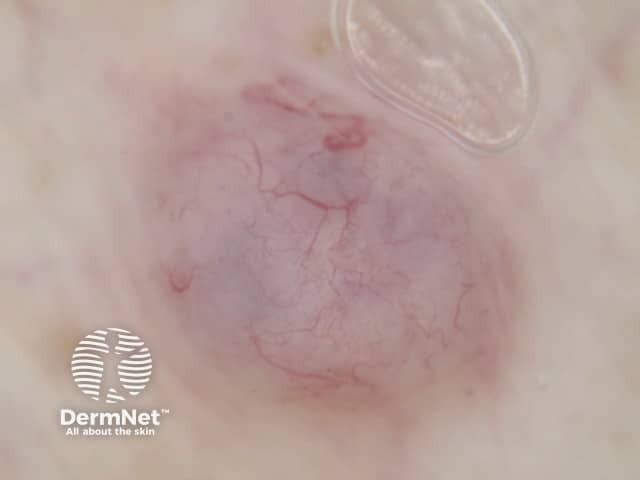
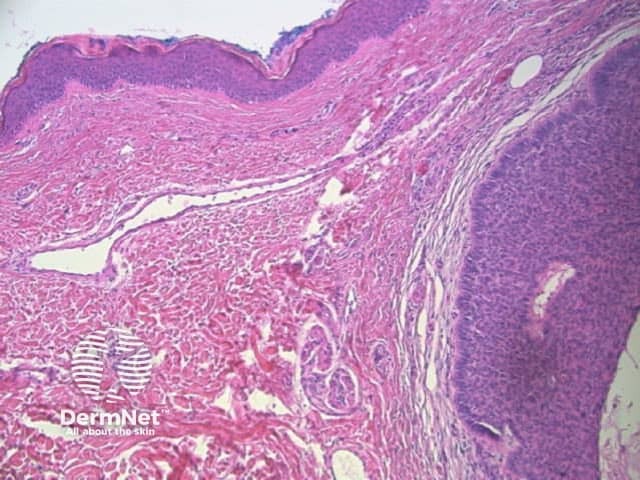
Review this article and correlate dermatoscopic and histologic features with each new lesion you excise.
See the DermNet bookstore.