Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Authors: Dr Malini Sivasaththivel, Box Hill Hospital, U.K. August 2022. Previously: Dr Mark Duffill, Dermatologist, New Zealand, 2001.
Introduction
Demographics
Causes
Clinical features
Variation in skin types
Complications
Diagnosis
Differential diagnoses
Treatment
Prevention
Outcome
Chronic superficial scaly dermatitis (CSSD) is a chronic dermatosis that is characterised by round or oval, red, scaly patches primarily on the limbs and trunk.
Also known as chronic superficial dermatitis, parapsoriasis, or digitate dermatosis, CSSD is relatively uncommon and can be subdivided into small (SPP) and large plaque parapsoriasis (LPP). Whilst SPP is thought to be a benign chronic condition, LPP is thought to be potentially premalignant and associated with a risk of progression to mycosis fungoides.
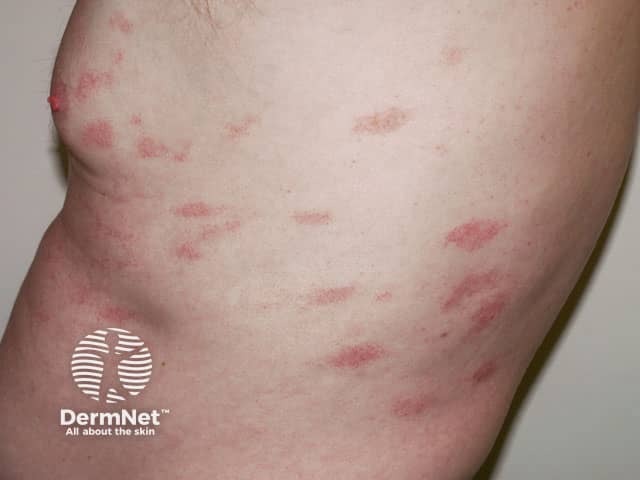
The finger-like pink scaly patches of CSSD (CSSD-patient1)
The finger-like pink scaly patches of CSSD (CSSD-patient1)
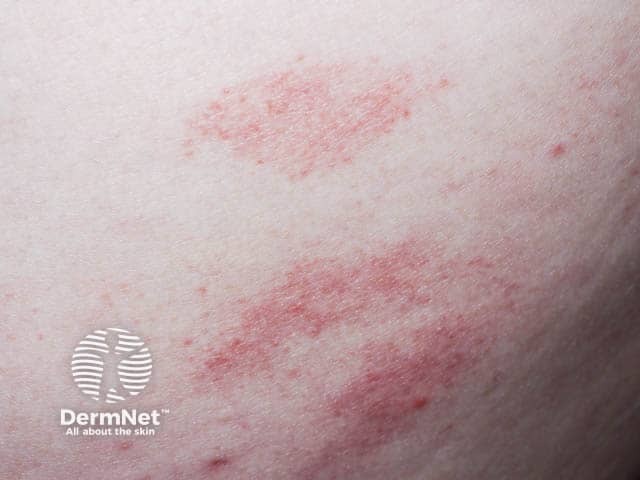
Digitate lesions of CSSD on the thorax and abdomen
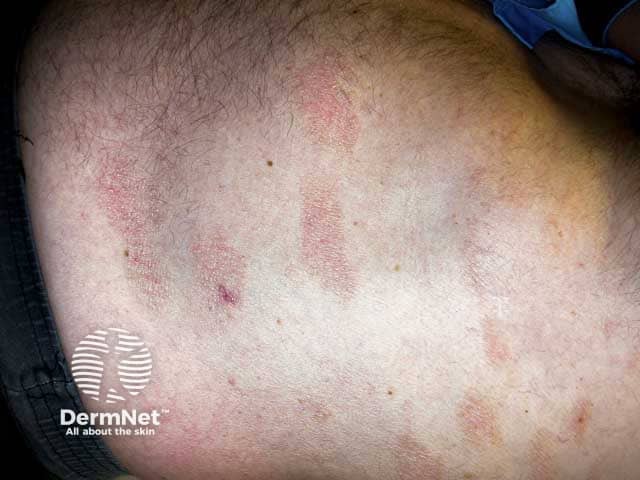
CSSD over the thighs (CSSD-patient3)
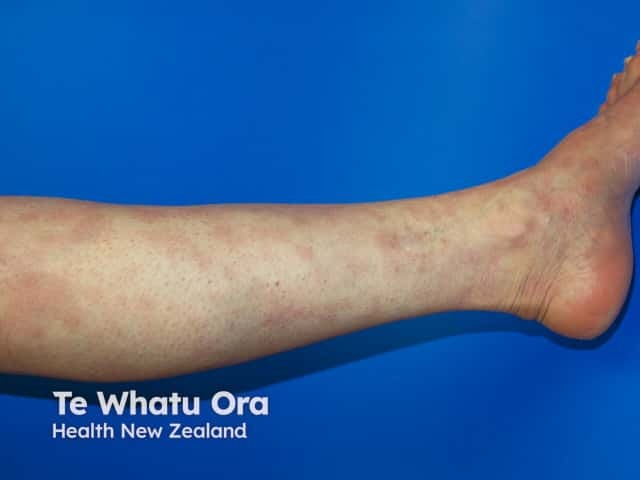
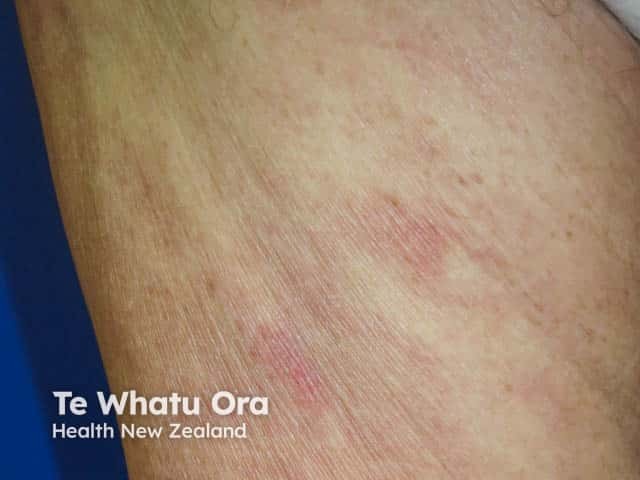
Patches of CSSD on the lower leg (CSSD-patient4)
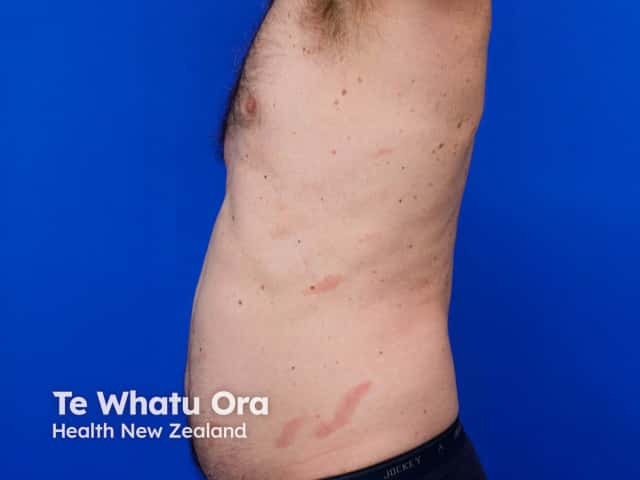
Finger-like patches of CSSD on the trunk (CSSD-patient5)
CSSD tends to occur in older individuals, with a male predominance. The exact incidence and prevalence of the condition is unknown.
The cause of chronic superficial scaly dermatitis is not known.
One hypothesis for its development is that it is a precursor to mycosis fungoides or an early form of the disease. The lesions are mainly comprised of clonal CD4+ T-cells, though the presence of T-cell clones does not appear to increase risk of malignancy.
Another hypothesis is that CSSD may be associated with viral infections such as the Epstein-Barr virus, cytomegalovirus, and human herpesviruses (HHV6 and HHV8).
Chronic superficial scaly dermatitis begins with one or more red, slightly scaly patches mainly on the limbs and trunk.
The patches may be:
Once the lesions resolve they may recur in the same or adjacent region.
SPP lesions are often 2–5 centimetres in diameter whilst LPP is more than 5 centimetres in diameter. Additionally, LPP may be associated with skin atrophy, telangiectasia, and mottled hyperpigmentation.
CSSD immunostaining also reveals a mature T-cell phenotype made mostly of CD4+ cells with some polymerase chain reaction (PCR) studies showing a dominant clonal pattern of T-cells. The clonal density is noted to be 1–10% but this does not seem to determine propensity to transition to malignancy. Approximately 7.5% to 14% of LPP progress to mycosis fungoides, though most remain benign for many years.
Features are similar in all skin types.
CSSD is a benign condition, though it may resemble cutaneous T-cell lymphoma and have the ability to transform into mycosis fungoides. Hence individuals should be cognisant of changes in skin lesions such as colour, thickening, increase in scaling, crusting, or thinning.
The clinical and histological findings consistent with CSSD include:
A punch biopsy may be taken with histopathological findings of SPP such as:
LPP is histologically similar to SPP, but can also consist of:
There may also be atypical or haloed lymphocytes and histological findings may be similar to that seen in mycosis fungoides.
Investigations such as nuclear contour studies, immunohistochemistry, PCR, and T-cell receptor gene rearrangement studies can aid in identifying atypical lymphocytes and hence show which LPP may become mycosis fungoides. However, these methods are not completely accurate. It is thought that earlier genotypic analysis of T-cell receptor rearrangement is the gold standard of all diagnostic tests enabling differentiation between benign and malignant T-cell infiltration.
Parapsoriasis does not require treatment but may be treated if symptomatic or if there is cosmetic concern.
There is no known way of preventing CSSD.
The long-term outcome of CSSD is variable. Most cases are present for a person’s lifetime with minor fluctuations, but spontaneous resolution may occur in some individuals.
There are reports of individuals developing mycosis fungoides and hence skin checks every 3–6 months and subsequently yearly with biopsies of suspicious lesions should be conducted. Elevated risk of thromboembolism, acute myocardial infarction, stroke, and cancer including non-Hodgkin lymphoma have been reported with CSSD.