Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author: Dr Audrey Ho, Resident, Singapore General Hospital, Singapore (2022).
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Dosing and administration
Benefits
Side-effects
Contraindications
In September 2022, spesolimab was approved by the US Food and Drug Administration (FDA) as a biological treatment for generalised pustular psoriasis flares (Spevigo®).
In October 2021, the European Medicines Agency (EMA) validated the marketing authorisation application, which is under review.
Spesolimab is approved for use in generalised pustular psoriasis (GPP).
There are also trials evaluating the efficacy of spesolimab for palmoplantar pustulosis. A pilot study (Mrowietz et al, 2021) found that spesolimab was well tolerated but did not meet the primary endpoint for efficacy in treating palmoplantar pustulosis. A phase IIb trial is underway.
There are case reports of successful treatment for severe pyoderma gangrenosum and acute generalised exanthematous pustulosis (AGEP), and acute febrile neutrophilic dermatosis (Sweet syndrome).
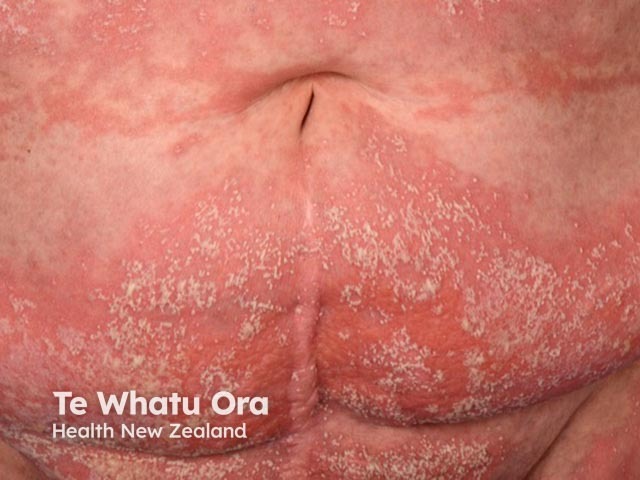
Sheets of pustules coalescing into lakes on the abdomen in generalised pustular psoriasis
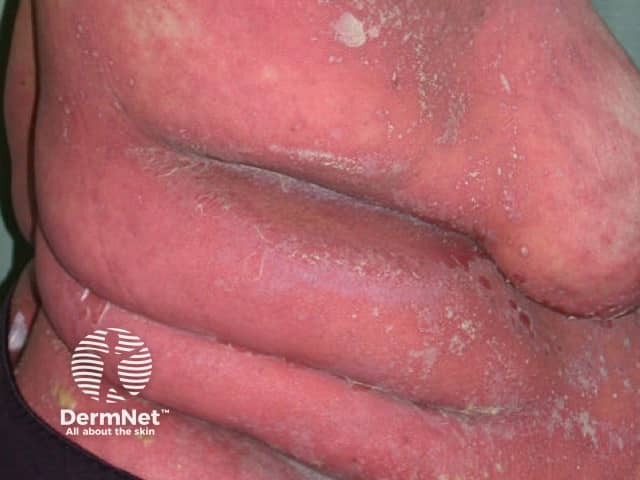
Erythrodermic generalised pustular psoriasis
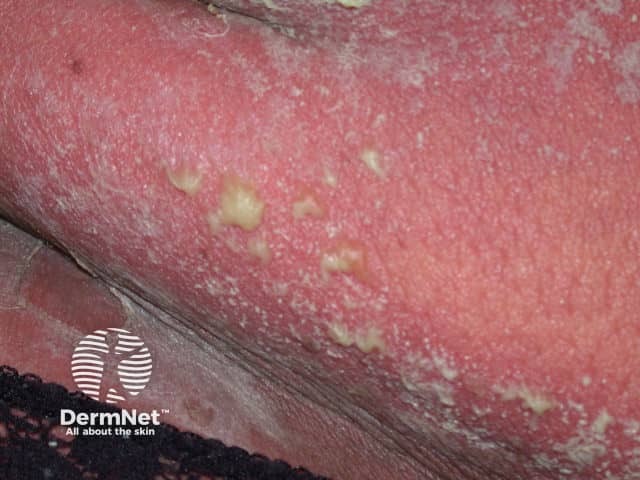
Superfical pustules in generalised pustular psoriasis
More images of generalised pustular psoriasis
Spesolimab is a humanised monoclonal antibody that binds to interleukin 36 (IL-36) receptors and prevents binding of IL-36, preventing activation of proinflammatory pathways.
As the IL-36 pathway is integral in the pathogenesis of GPP, its downregulation is proposed to help relieve GPP flares.
IL-36 has also been shown to play a role in the pathogenesis of palmoplantar pustulosis, and the use of spesolimab in this context is being investigated.
Effisayil-1, a phase II, multi-centre, randomised, double-blind, placebo-controlled trial (Bachelez et al, 2021), examined the efficacy of spesolimab in 53 patients with moderate-to-severe generalised pustular psoriasis (GPP) flares.
Reported side effects in patients given spesolimab include:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).