Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Linda Chan, Senior Resident Medical Officer, Department of Dermatology, Concord General Repatriation Hospital, Sydney, Australia. DermNet New Zealand Editor in Chief: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy Editor: Gus Mitchell. October 2017.
Introduction
Coagulase-negative staphylococcal skin conditions
Demographics
Symptoms and signs
Diagnosis
Central nervous system shunt infections
Causes of infection
Treatment
Outcome
The human skin is the first line of defence between the body and the outside world. As a result, the skin is physiologically colonised by a host of microorganisms, including at least 47 species of coagulase-negative staphylococci [1]. Coagulase-negative staphylococci are gram-positive, aerobic organisms distinguished from the closely related Staphylococcus aureus by the group's inability to form coagulase, an enzyme that promotes thrombus formation via the conversion of fibrinogen into fibrin [2]. They were first identified by the microbiologists Louis Pasteur and Alexander Ogston in the 1880s [1].
Coagulase-negative staphylococci are an important part of normal skin microbiota, and they also colonise mucous membranes in adults and children from a few weeks of age [1]. Staphylococci prefer humid areas and are therefore commonly found in the axillae, gluteal, and inguinal regions as well as anterior nares and the conjunctiva [3].
Below is a list of common coagulase-negative staphylococcal species and their preferred sites of colonisation.
Until two decades ago, coagulase-negative staphylococci were commonly perceived as contaminants in clinical specimens. Now, with the increasing use of implanted medical equipment, they have become leading pathogens for nosocomial infections owing to their ability to form biofilms on foreign material [1,2]. The S. epidermidis group of coagulase-negative staphylococci are of particular importance.
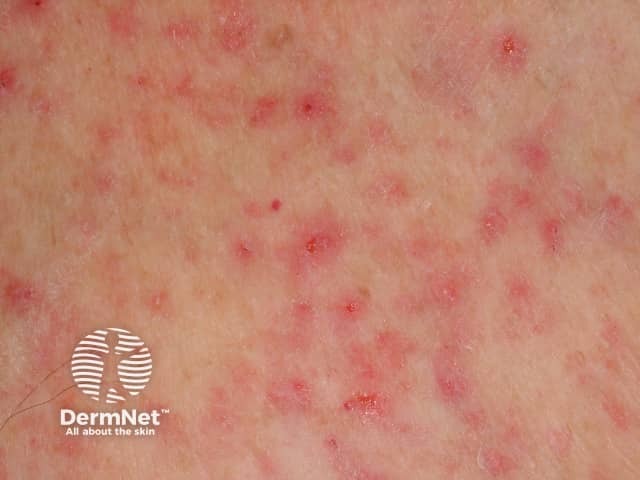
Miliaria
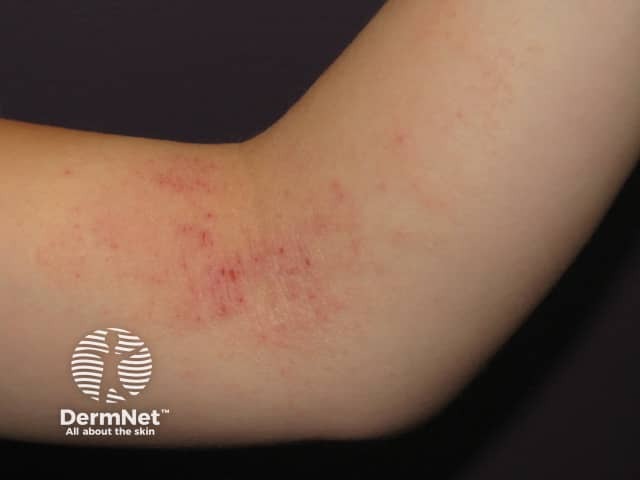
Atopic eczema
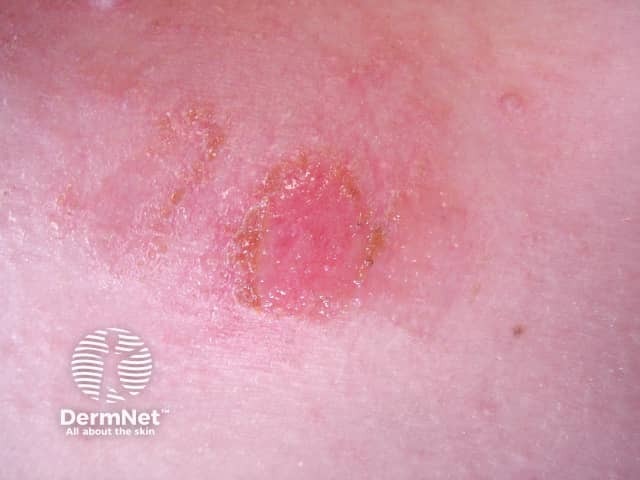
Impetigo
S. epidermidis can induce miliaria, a disorder characterised by the retention of sweat within the eccrine glands. Skin biopsies have shown that periodic acid–Schiff (PAS)–positive material tends to block the upper eccrine sweat ducts. Miliaria is associated with:
Miliaria is not associated with non-EPS producing strains of S. epidermidis or another coagulase-negative staphylococcus, such as S. haemolyticus and S. hominis. Of note, up to 62% of S. epidermidis strains on the forehead and back produce EPS [4,5].
Occluded sweat ducts may also lead to hyperhidrosis and anhidrosis, which may occur in chronic dermatoses such as psoriasis, atopic dermatitis and systemic sclerosis [5].
Coagulase-negative staphylococci are implicated in the 'double-hit' phenomenon, a theory used to explain the cause of atopic dermatitis. The abnormal stratum corneum (skin surface) is attributed to the combined effects of an abnormal FLG gene and an unknown environmental trigger. The specific environmental trigger may be subclinical miliaria, as PAS-positive material has been discovered in the eccrine ducts of patients with atopic dermatitis. The PAS-positive material may arise from S aureus and EPS–producing strains of S. epidermidis. Rather than causing the usual miliaria lesions, in patients with a FLG defect, occlusion of the eccrine ducts may trigger a flare of atopic dermatitis by activating the innate immune system [3].
In the study described above, all skin samples from patients with atopic dermatitis contained drug-resistant staphylococci. S. aureus accounted for 42% and S. epidermidis in 20%, and all were positive for EPS and biofilms [3].
Coagulase-negative staphylococci are competitors against S. aureus, a common pathogen, on the surface of normal skin. All organisms use quorum–sensing systems in which virulence factors are only expressed in a dense population of bacteria that is adapting to a changing environment. The quorum-sensing system for staphylococci is known as the accessory gene regulator (agr) system. Each staphylococcal subspecies has pheromones that can block the agr system of foreign species.
Skin samples from patients with atopic dermatitis patients are colonised with greater proportion of S. aureus than healthy controls, which are colonised with S. epidermidis [3]. Skin colonisation by S. epidermidis may confer protection against atopic dermatitis, particularly in patients with the FLG gene defect.
Despite their abundance on the skin, coagulase-negative staphylococci rarely cause disease in intact skin. The main risk factor for coagulase-negative staphylococcal infection is a medical implant on which the organism can colonise, proliferate, and gain access to the systemic circulation [1,2,8].
Specific risk factors for coagulase-negative staphylococcal infection are:
Clinical signs such as fever, hypotension and leukocytosis are helpful in differentiating between true infections from coagulase-negative staphylococcal contamination [2].
Certain microbiological findings can support a diagnosis of infection, as opposed to contamination.
Coagulase-negative staphylococci are identified in the laboratory.
Coagulase-negative staphylococci are more often cultured from superficial incisional wounds than from deeper wounds. A diagnosis is obtained by finding they are the predominant microorganisms or by repeated isolation of the same organism in serial cultures [9].
Two sets of blood cultures should be obtained in a patient with fever and signs of bacteraemia [8]. If the patient has an indwelling central catheter, one of the blood cultures should be collected through the catheter [10].
Diagnosis is achieved by a positive culture of the catheter tip, which is considered the gold standard [10].
Prosthetic vascular graft infection is most commonly associated with grafts distal to the inguinal region (1–6%). Infection can occur within 30 days of grafting, but more commonly occurs months to years later. It is diagnosed by physical examination and imaging, which shows sinus tracts or pseudo-aneurysms (pockets of blood in the bloodstream) at the site of vascular anastomosis (a connection between blood vessels) [8].
Endocarditis is due to S. epidermidis in 15–40% cases. Diagnosis is achieved via repeated positive blood cultures with suggestive transthoracic echo findings (80% will have valve dysfunction and intracardiac abscess), usually > 12 months after valve placement.
This form of endocarditis is rarely caused by coagulase-negative staphylococci, occurring in only 8% of cases of endocarditis. It is due to haematogenous seeding of previously damaged or malformed heart valves and endocardium [8].
Coagulase-negative staphylococcus, predominantly S. epidermidis, is the culprit pathogen in 25% of pacemaker infections. About 25% of infections occur within 1–2 months of insertion of the device, due to inoculation at the time of placement of the device. Symptoms include inflammation at the pacer pocket site, systemic bacteraemia, and right-sided endocarditis.
Diagnosis is achieved via culture of the generator pocket and of the device itself, or by multiple positive sequential blood cultures with the same strain of bacteria [8].
Coagulase-negative staphylococci are usually are inoculated at the time of surgery, but remain indolent and is only present between 3 months and two years later. S. epidermidis is the main pathogen in these infections with a few cases being caused by S. lugdunensis.
Diagnosis is determined through reports of unexplained joint pain together with a high erythrocyte sedimentation rate, positive bone scan findings and culture of the prosthesis. There can be culture-negative prosthetic joint infections, which manifest as aseptic joint loosening [8].
Coagulase-negative staphylococci are responsible for more than 50% of central nervous system shunt infections. Risk factors are:
Symptoms include unexplained fevers within two months of shunt placement or shunt dysfunction. Definitive diagnosis is determined through positive culture from cerebrospinal fluid drawn from the shunt or ventricles, or positive culture of the shunt [8].
Coagulase-negative staphylococci gain entry through breached skin surfaces, commonly during medical or nursing procedures. The key mechanism is the ability of the bacteria to form biofilms on the surfaces of implanted medical equipment, where the bacteria replicate and disseminate within the systemic circulation [9].
The key steps are:
When treating coagulase-negative staphylococcal infections, the clinician should consider the:
The mainstay of treatment is appropriate systemic antibiotic therapy and removal of the culprit implant [8].
Approximately 90% of infections are resistant to penicillin. Vancomycin is the drug of choice. If the organism is confirmed to be susceptible to methicillin, vancomycin can be replaced by lactamase–resistant penicillin or a first– or second-generation cephalosporin. Newer antibiotics with activity against coagulase-negative staphylococci are daptomycin, linezolid, clindamycin, telavancin, tedizolid and dalbavancin [1,9]. Gentamicin or rifampicin can be added for deep-seated infections.
The duration of treatment depends on the site of infection. (Detailed information on the duration of treatment can be found in guidelines published by the Infectious Diseases Society of America).
Other treatments
Due to the correlation between mucosa colonisation and subsequent bacteraemia, treatment can be given to decrease mucosal colonisation with coagulase-negative staphylococci. One suggested the approach is topical mupirocin for nasal decolonisation and an oral glycopeptide, such as ramoplanin, for intestinal decolonisation [11].
Coagulase-negative staphylococcal bacteraemia is a serious medical condition associated with significant morbidity and mortality.