Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Vanessa Ngan, Staff Writer, 2003. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Updated: Dr Sachin Manohar Shetty, Consultant Dermatologist, Puttur Skin Clinic, Puttur, Karnataka State, India, 2018. Revised September 2020. Copy edited by Gus Mitchell. September 2020. DermNet revision January 2021.
Introduction
Demographics
Causes
Clinical features
Complications
Diagnosis
Differential diagnoses
Treatment
Outcome
Prevention
Leprosy, also called Hansen disease, is a chronic bacterial infection primarily affecting the skin and peripheral nerves usually caused by Mycobacterium leprae. The form the disease takes depends on the person’s immune response to the infection.
Leprosy can affect people of all races anywhere in the world. However, it is most common in warm, wet areas of the tropics and subtropics. In 2017, over 200,000 new cases of leprosy were registered world-wide. Worldwide prevalence is reported to be around 5.5 million, with 80% of these cases found in 5 countries: India, Indonesia, Myanmar, Brazil, and Nigeria. In New Zealand, there were 38 reported cases in the decade 2004-2013 with the majority of cases having lived in the Western Pacific region or Southeast Asia: most cases came from Samoa. A small number of new cases are seen annually in Australia affecting the indigenous population and migrants from endemic areas.
Infection can present at any age. There are two age peaks; 10–14 years of age, and 35–44 years. It is rarely seen in infants and young children.
The mode of transmission of infection remains controversial. It is believed to be spread by breathing airborne droplets from affected individuals when they sneeze or cough. In 80% of new cases, there is a clear history of prolonged contact with an untreated person suffering from leprosy. The majority of exposed individuals will not develop symptoms. The incubation period is long; on average around 5 years, range 6 months to 20 years. Symptoms are usually mild initially and are only noticed years after exposure.
Armadillos are an important source of infection in Brazil and southern states of the US. People who hunt, kill, process, or eat armadillo meat are at high risk for infection with M. leprae.
Several genes have been identified which increase the susceptibility to infection with M. leprae. Up to 95% of the world population have been estimated to not be genetically susceptible to infection with M. leprae.
Leprosy is nearly always caused by M. leprae, an intracellular acid-fast bacillus related to Mycobacterium tuberculosis and Mycobacterium ulcerans. M. leprae grows best at cool temperatures, explaining its predilection to affect skin and peripheral superficial nerves. It divides very slowly and takes years to reach a number sufficient to show signs of infection.
Mycobacterium lepromatosis was identified by genome sequencing of isolates in Mexico and has subsequently been identified in many other countries. It is believed to have reached the Americas with populations migrating from Asia across the Bering Strait, whereas M. leprae travelled with colonists from the Old World.
The clinical manifestations of leprosy depend on the immune response to M. leprae/lepromatosis and range over a spectrum from multibacillary lepromatous leprosy (LL) showing limited or low immunity to M. leprae/lepromatosis, to paucibacillary tuberculoid leprosy (TT) with a strong immune response.
In over 90% of patients, the first symptom noticed is numbness. Temperature is the first sensation lost, followed by light touch, pain, and then deep pressure. This may precede the development of cutaneous lesions by years. The initial skin lesions are usually of the indeterminate type, presenting as a solitary or small number of hypopigmented patches before evolving into borderline tuberculoid or lepromatous types.
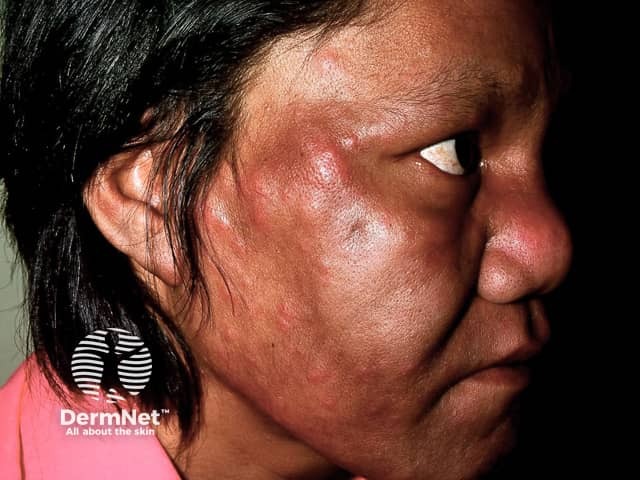
Tuberculoid (TT) leprosy is the paucibacillary form defined clinically by:
Well-defined erythematous plaque on the cheek in skin of colour
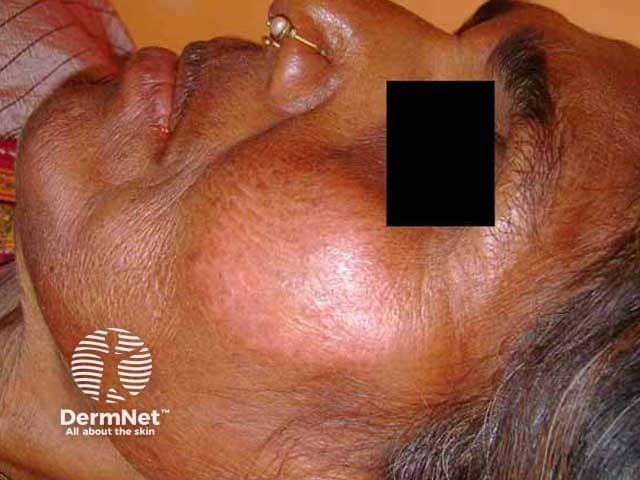
Borderline tuberculoid (BT) leprosy presents with:
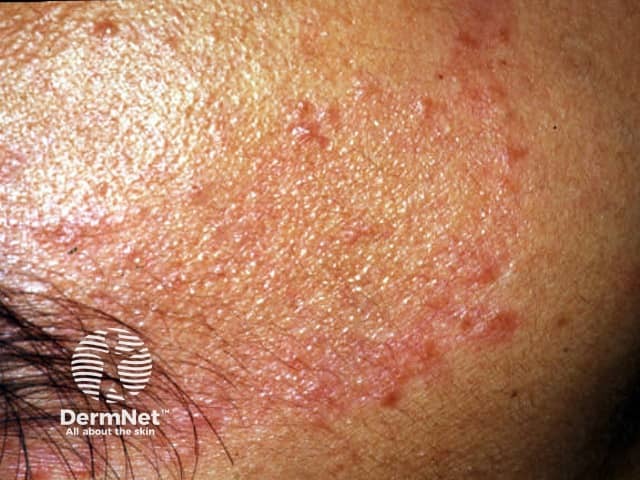
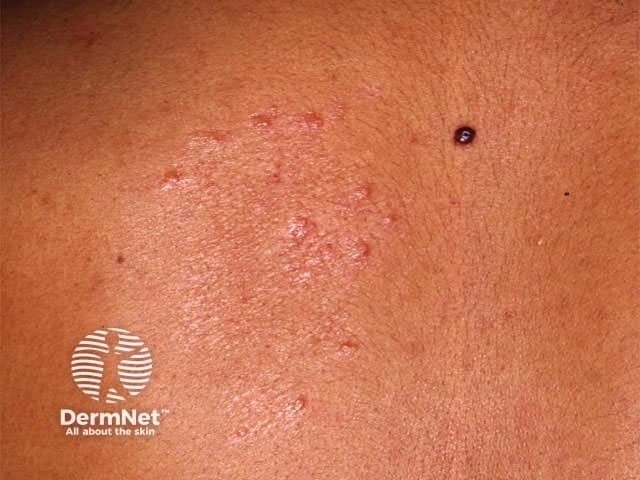
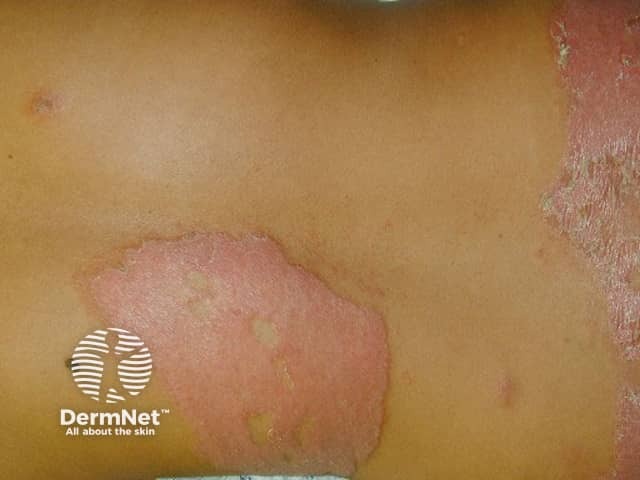
BT leprosy with type 1 reaction
Borderline borderline (BB) leprosy is a rarely seen, transient, unstable form of leprosy defined by:
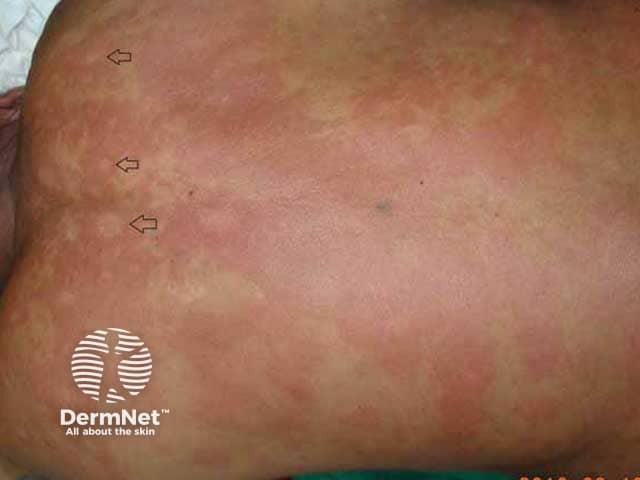
Red lesions of variable size. Characteristic 'Swiss cheese' appearance (black arrows)
Borderline lepromatous (BL) leprosy is characterised by:
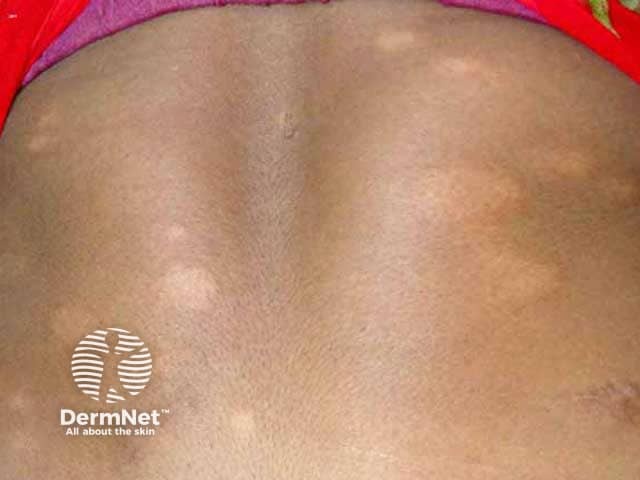
Asymmetrical distribution of erythematous infiltrated plaques in skin of colour
Lepromatous (LL) leprosy is the multibacillary form defined by:
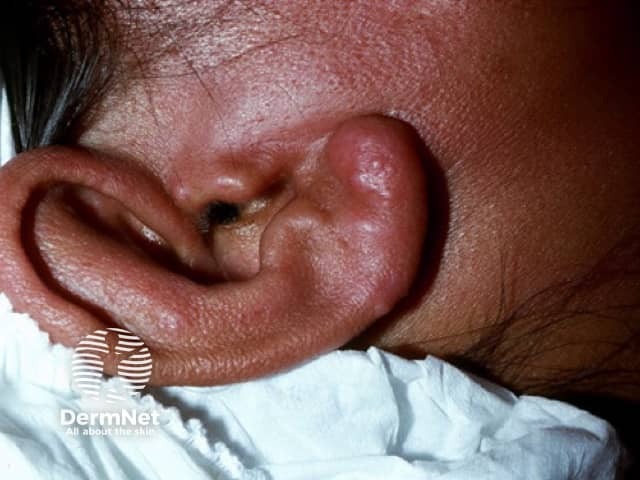
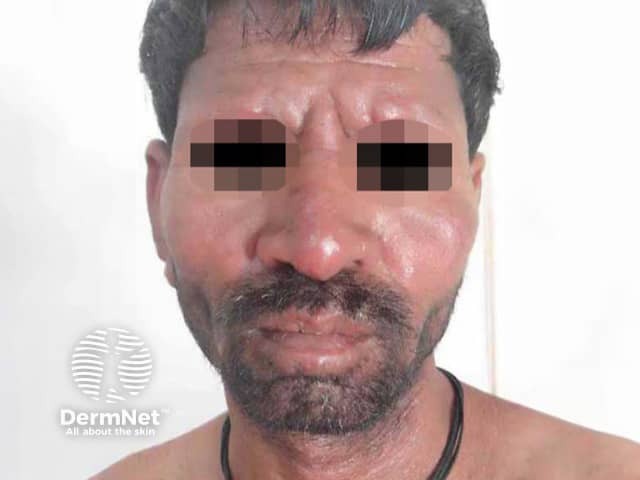
Leonine facies with loss of eyebrows and infiltrated facial skin
A number of clinical variants of lepromatous leprosy are described.
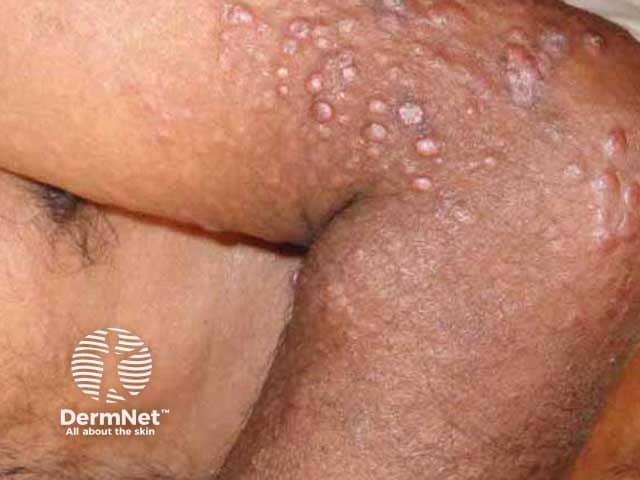
Dome-shaped papules and nodules in skin of colour
Pure neural leprosy (PNL) is common in India and Nepal. It presents with only peripheral nerve tenderness and thickening without skin lesions. However the nerve damage can result in loss of sensation and hence trophic ulcers. Diagnosis is difficult and requires nerve biopsy.
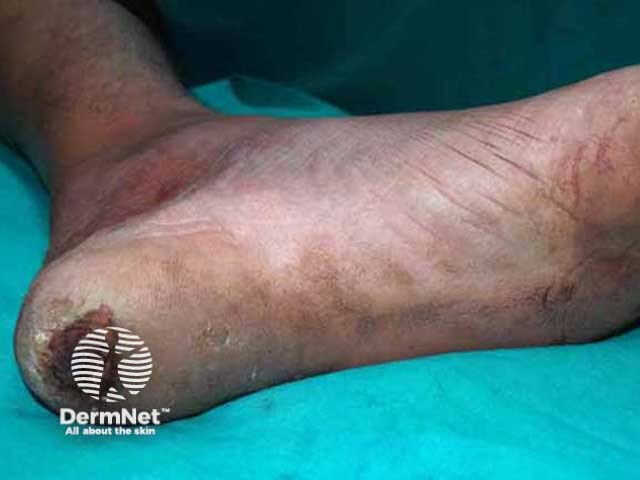
Trophic ulcer on the heel
Lepra reactions occur in 30–50% of patients with leprosy. They may occur before, or more often, after the start of treatment. These are sudden responses resulting from the release of immunologically active bacilli or its products leading to localised or systemic symptoms and signs. Such reactions are responsible for most of the nerve damage, deformity, and disability.
Leprosy has very characteristic clinical features, and dermoscopy is being used more often to aid clinical diagnosis.
Diagnosis should be confirmed by one of the following investigations.
The potential differential diagnosis list for leprosy is long and depends on the type and clinical features.
The treatment of leprosy aims to stop active infection and minimise complications and deformity. Residual disabilities may require corrective reconstructive surgery to allow day-to-day activity.
Most endemic countries follow the WHO recommended multi-drug therapy (MDT) of antibiotics; the combination of drugs selected and duration of treatment depends on the type of leprosy. The 2018 WHO guidelines recommend three drugs including clofazimine for 6 months in paucibacillary leprosy and 12 months for multibacillary disease.
First-line antibiotics used in the treatment of leprosy are dapsone, rifampicin and clofazimine. Other drug options include ofloxacin, moxifloxacin, minocycline, clarithromycin, rifapentine, and diarylquinolone. Vaccines and other forms of immunotherapy are being trialled.
Once appropriate treatment has been commenced, the skin lesions slowly subside. However, nerve damage cannot be reversed.
Post-exposure prophylaxis using rifampicin reduces the development of paucibacillary leprosy by 50%.