Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Authors: Vanessa Ngan, Staff Writer, 2003. Updated: Dr Kelvin Truong, Dermatology Research Fellow, Westmead Hospital, Sydney, Australia. Copy edited by Gus Mitchell. October 2021
Introduction Demographics Contraindications and precautions More information Benefits Disadvantages Side effects and risks
Mycophenolate mofetil is the prodrug form of mycophenolic acid, a potent immunosuppressive drug. Enteric-coated mycophenolate sodium is another form of mycophenolic acid used in clinical practice.
Mycophenolate mofetil is an immunosuppressant approved for to prevent rejection of solid organ (kidney, liver, heart) transplants.
Off-label use of mycophenolate mofetil is widespread in dermatology.
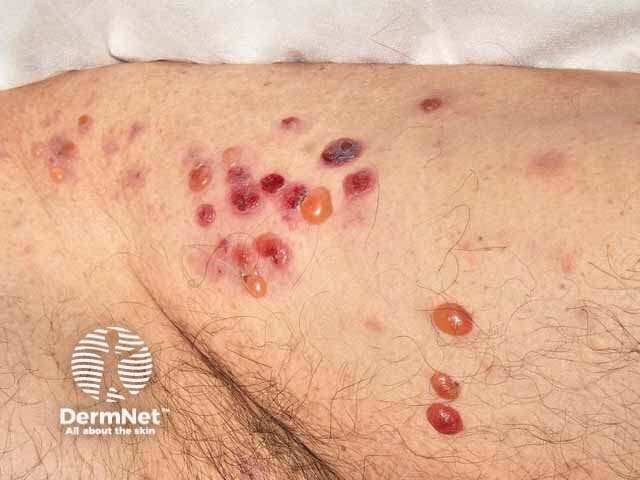
Bullous pemphigoid
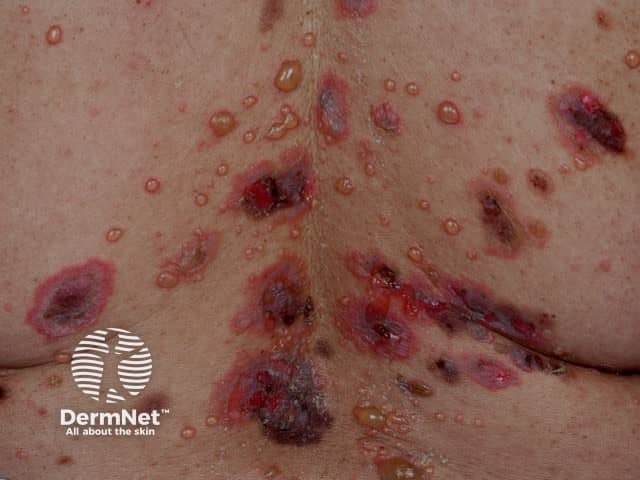
Pemphigus vulgaris
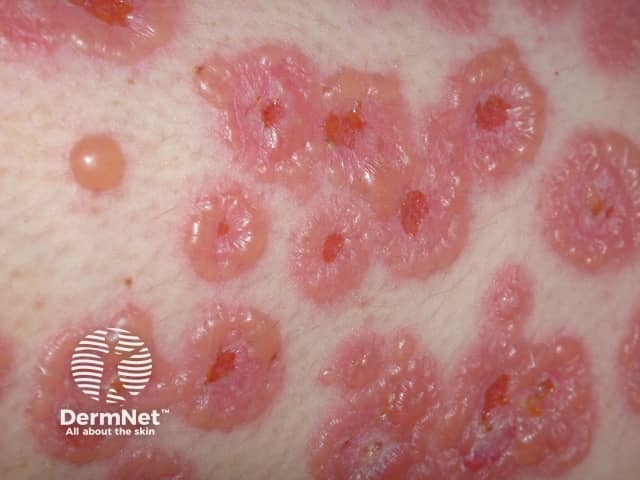
Linear IgA bullous disease
Mycophenolate mofetil is available in oral and intravenous formulations.
A typical starting dose used in dermatology is 250 mg twice daily. If no improvement is observed after one month of therapy, the dose may be increased by 500 milligram increments up to a maximum of 3 g per day.
Dose reduction is required with chronic renal insufficiency as mycophenolic acid is mostly excreted via the kidneys.
Mycophenolate mofetil is often used in combination with other immunosuppressive drugs [see Drug-induced immunosuppression]
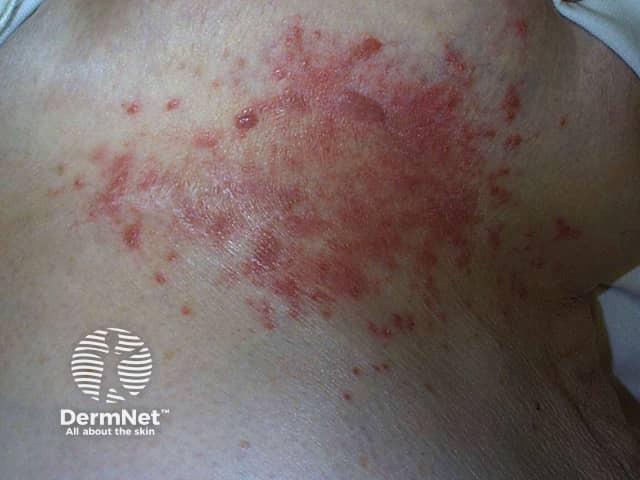
Candida intertrigo
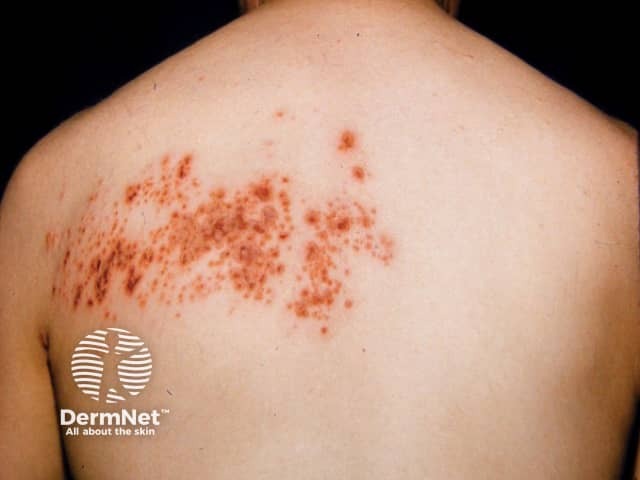
Herpes zoster
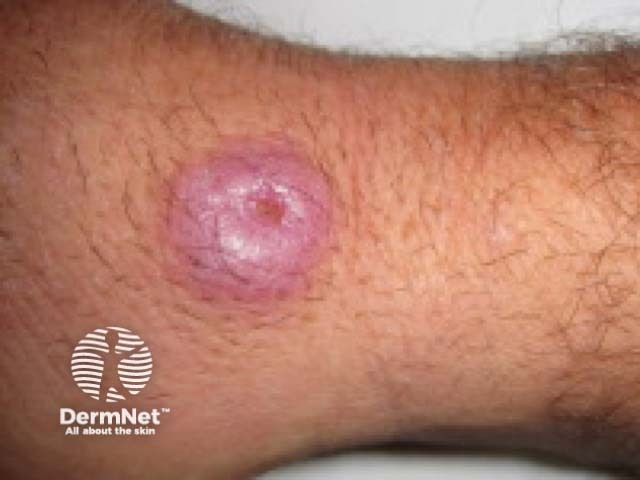
Atypical mycobacterial infection
Mycophenolate mofetil should be ceased if pregnancy occurs or:
Bone marrow suppression, if mild, usually recovers with dose reduction or cessation of the mycophenolate mofetil.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).