Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: August, 2025
Author: Jonathan Haddad, King’s College London, England (2025)
Peer reviewed by: Nancy Huang (MBChB), DermNet Medical Writer, NZ; Dr Iaza Hussain, Chesterfield Royal Hospital Foundation Trust, England (2025)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department.
Introduction
Uses
Contradictions and cautions
More information
Pretreatment
Benefits
Side effects and risks
Further information
Ritlecitinib is an oral kinase inhibitor that selectively inhibits Janus kinase 3 (JAK3) and the TEC-family kinases. Receiving approval from several medical regulatory bodies including the FDA (United States), EMA (European Union), MHRA (United Kingdom), and TGA (Australia), it is indicated for the treatment of severe alopecia areata.
Ritlecitinib is marketed under the brand name Litfulo® and is manufactured by Pfizer.
In 2023 and 2024, ritlecitinib received approval from several countries for the treatment of severe alopecia areata in adults and adolescents aged 12 years and older. At this time, ritlecitinib is not approved for any other indications.
Ritlecitinib is also under investigation by Pfizer for potential use in other immune-mediated disorders such as vitiligo, Crohn disease, and ulcerative colitis.
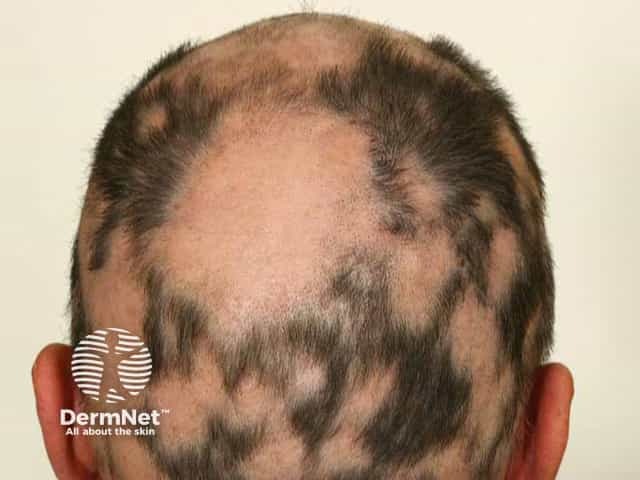
Patchy alopecia areata with a SALT score in excess of 50
Many of the contraindications and cautions related to ritlecitinib are due to its immunosuppressive effects, and subsequent increased risk of infections.
Contraindications:
Precautions:
Ritlecitinib is a highly selective, irreversible inhibitor of two enzyme systems: Janus kinase 3 (JAK3) and the TEC (tyrosine kinase expressed in hepatocellular carcinoma) family of kinases.
JAK3 belongs to the Janus kinase family, alongside JAK1, JAK2, and tyrosine kinase 2.
The TEC family consists of five intracellular tyrosine kinases: TEC, BTK Bruton tyrosine kinase, ITK (inducible T-cell kinase), RLK/TXK (resting lymphocyte kinase), and BMX/ETK (bone marrow kinase). Both families play critical roles in regulating immune responses.
Alopecia areata is an autoimmune disorder in which the immune system targets hair follicles, resulting in hair loss. By inhibiting JAK3 and TEC family kinases, ritlecitinib blocks key inflammatory pathways implicated in the immune-mediated damage of hair follicles observed in alopecia areata.
Ritlecitinib is indicated in the United Kingdom for people with alopecia areata affecting more than 50% of the scalp (SALT score 50), or with less extensive disease associated with psychological upset or with brow or lash alopecia or rapidly advancing disease. Responses are assessed after 36 weeks of therapy. Almost half of recipients can be expected to achieve a SALT score reducing from over 50% to 20% or under. Of these responders, about a half can be expected to have a complete regrowth. Response rates may be lower in those with a long duration of disease.
Ritlecitinib is administered as a once-daily oral capsule, with a recommended dose of 50 mg. It can be taken with or without food.
Before starting ritlecitinib, patients should be screened for infection, including tuberculosis. If possible, any infections should be treated before starting ritlecitinib. Patients should also receive all recommended vaccinations before starting treatment.
Live vaccines should be avoided during or just before initiating treatment. See: Immunisation in immunosuppressed dermatology patients.
Patients should be advised that ritlecitinib will be discontinued if it is ineffective or causes significant adverse effects. Furthermore, patients should be informed that any hair regrowth will likely be lost upon discontinuation of treatment.
Platelet and lymphocyte count:
Infection:
Routine skin examinations:
Common:
Boxed warnings:
The Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMA) have issued guidance to help clinicians reduce the risk of serious side effects associated with all JAK inhibitors. These include cancer, major cardiovascular events, serious infection and blood clots.
It is recommended to avoid JAK inhibitors, unless no alternatives exist, in patients who are:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).