Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Nick Turnbull, Dermatology Registrar, London, United Kingdom, 2011.
Introduction - EGFR inhibitors Introduction - protein kinase inhibitors How they work Cutaneous side effects Side effects
Epidermal growth factor receptor (EGFR) inhibitors are a group of medicines developed to treat a wide range of cancers. Overexpression of EGFR by about 30% of cancers is one reason for their excessive cell proliferation and tumour growth.
Although effective in the treatment of many cancers, EGFR inhibitors often result in adverse reactions in the skin, which occur in at least half of treated patients. In some cases, an adverse skin reaction indicates the medicine is effective in treating the tumour.
As treatment with these medicines may be longterm, the benefits versus the side effects will need to be considered case-by-case.
Protein kinases are enzymes within the cell involved in cellular proliferation. Protein kinase inhibitors reduce cell proliferation. They target the amino acids tyrosine, threonine, and serine. Some EGFR inhibitors are also protein kinase inhibitors.
EGFR inhibitors target EGFR on the outside of the cell. Protein kinase inhibitors target specific protein kinases on the inside of the cell. The receptors expressed by the cancer determine which drug is likely to be of benefit.
EGFR and protein kinase inhibitors are less toxic than traditional chemotherapies. Specific drugs target different types of cell and may be monoclonal antibodies with the suffix -mab or small molecules with the suffix -nib.
Examples of EGFR inhibitors and protein kinase inhibitors |
||
|---|---|---|
Drug name |
Molecular target |
Example Indications |
Monoclonal antibody |
||
Cetuximab |
KRAS |
Colorectal cancer |
Panitumumab |
KRAS |
Colorectal cancer |
Small molecule |
||
BCR-ABL, PDGFR, and c-KIT |
Chronic myeloid leukaemia (CML), |
|
Gefitinib |
EGFR, HER1 |
Non-small cell lung cancer (NSCLC). |
Erlotinib |
EGFR |
Metastatic NSCLC |
Lapatinib |
EGFR, HER2/neu |
Breast cancer |
Osimertinib |
EGFR, T790M/neu |
Non-small cell lung cancer |
Vandetanib |
EGFR, VEGFR, RET tyrosine kinase |
Thyroid tumour |
Sorafenib |
VEGFR, PDGFR, RAF |
Renal cell carcinoma |
Sunitinib |
PDGFR, VEGFR, KIT and others |
Renal cell carcinoma |
EGFR = epidermal growth factor receptor; PDGFR = platelet-derived growth factor receptor; VEGFR = vascular endothelial growth factor receptor
BRAF is a threonine kinase protein and the BRAF inhibitors, vemurafenib and dabrafenib, are effective in the treatment of metastatic melanoma with the V600E BRAF gene mutation.
Many more EGFR and protein kinase inhibitors are under development.
The most common cutaneous side effects of the monoclonal antibody EGFR inhibitors are:
Folliculitis (inflamed hair follicles) occurs in up to 40-85% of patients. It is an early event, usually seen within the first ten days of treatment. Sterile inflammatory papules and pustules are seen predominantly on the T-zone of the face, but they can be more widespread involving the chest and back. Occasionally the scalp and pubic regions are involved or rarely the entire body.
Folliculitis is especially frequent and often severe with cetuximab. The intensity of the folliculitis can fluctuate even when continuing to take the drug.
The folliculitis is often managed with:
If folliculitis is severe, the drug may need to be stopped, and it will resolve. The incidence and severity of the EGFR inhibitor-induced follicular reactions are reduced by half by pre-treating with doxycycline or minocycline. These tetracycline medications can also be prescribed once the rash has occurred allowing continued treatment with the EGFR inhibitor drug.
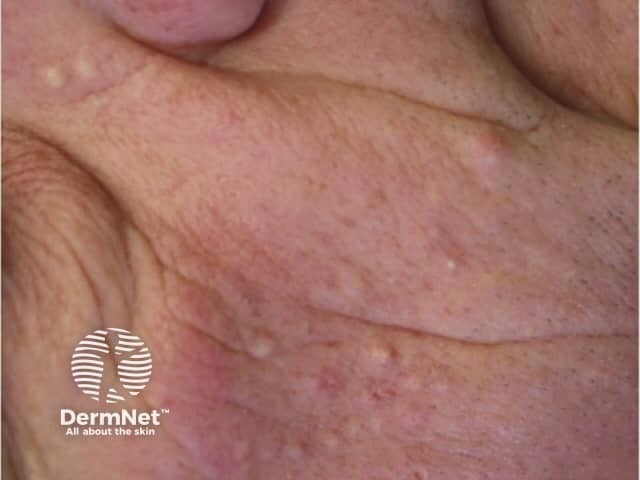
Folliculitis due to vemurafenib
Drug-induced alopecia (hair loss) is less common than folliculitis and tends to occur later, usually at 2-3 months. Patients may report their hair becoming fine and brittle. They may experience frontal-temporal hair loss similar to male pattern balding. This may or may not improve if the drug is stopped.
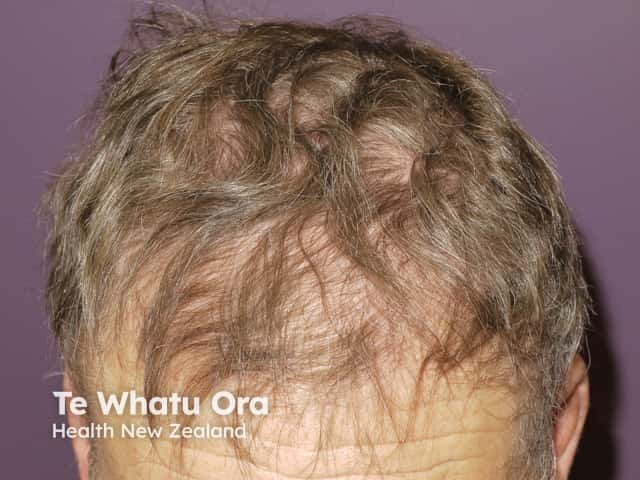
Alopecia due to vemurafenib
In contrast, patients often experience excessive facial hair and eyelash growth (hypertrichosis, trichomegaly).
Dry skin is common and treated with emollients. These drugs can cause pulpitis with painful fissures on the fingertips.
The dry skin sometimes resembles seborrhoeic dermatitis.
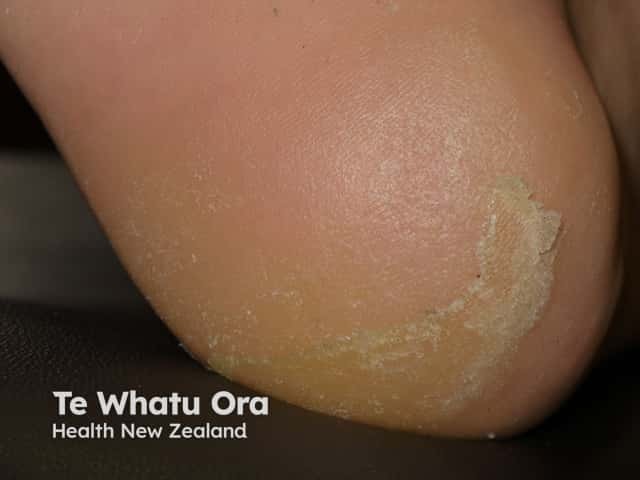
Keratoderma due to vemurafenib

Dry skin due to vemurafenib
Paronychia refers to painful inflammation of the nail folds. Fingers are more frequently affected than toes. Paronychia can sometimes resolve spontaneously even with continued use of the drug, but it clears quickly on stopping it.
Treatment involves avoiding trauma such as from tight shoes, avoiding excessive trimming or nailbiting, and wearing appropriate footwear. Topical steroids and antiseptics may help. It is essential to treat secondary bacterial and fungal infection.
Squamous cell carcinoma and multiple keratoacanthomas have been reported to arise in patients on sorafenib and other EGFR inhibitors.
The small molecule kinase inhibitors often block multiple enzymes meaning means there is some cross over in the actions and side effects of these drugs.
The most common side effects are:
The same side effects may also arise with monoclonal antibodies .
Imatinib induces a rash in a third of patients who start taking it, but this rash is generally self-limited. Imatinib has rarely been associated with vasculitis, Steven Johnson syndrome/TEN, and acute generalised exanthematous pustulosis (AGEP).
A red facial rash is frequently seen within the first two weeks of treatment with sorafenib. It can appear very much like seborrhoeic dermatitis and usually settles without treatment. It can be associated with scalp tingling or dysaesthesia.
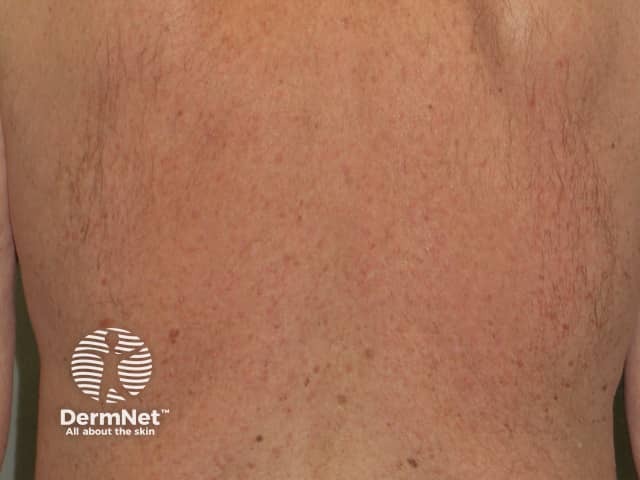
Rash due to vemurafenib
Swelling (oedema) of eyelids and face is common with imatinib and sunitinib. Oedema may also affect lower legs, lungs (pleural effusions) and brain (cerebral oedema).
Acral erythema (red hands and feet) is commonly seen with sorafenib and sunitinib. It presents as painful symmetrical red, swollen palms and soles. These drugs may also cause keratoderma/hyperkeratosis (excessive scale) and desquamation (peeling). Acral erythema tends to occur two to four weeks after starting treatment and is dose-dependent. It rapidly improves on stopping the drug and may not recur on restarting it.
The small molecule kinase inhibitors tend to cause less extensive but more scaly acral erythema than that induced by standard chemotherapy (hand-foot syndrome or palmar-plantar erythrodysaesthesia).
Preventative measures include appropriate footwear to prevent injury to pressure areas. Discomfort may necessitate brief interruption of treatment.
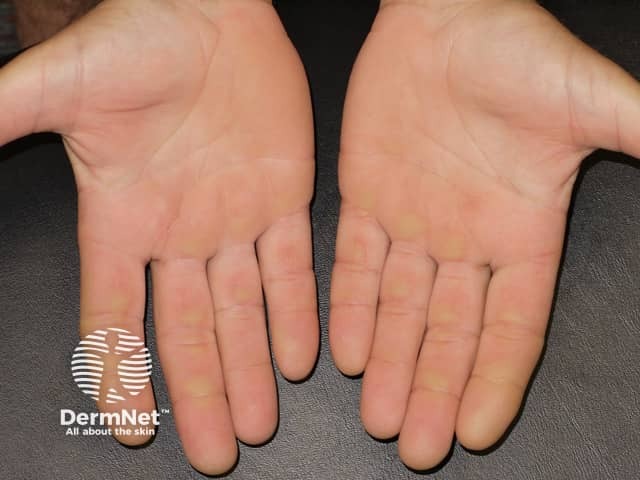
Painful red, callused palms due to protein kinase inhibitor
Painless distal splinter haemorrhages are common, particularly affecting the fingernails. It has been suggested that they are due to inhibition of vascular endothelial growth factor receptor (VEGFR). VEGFR is thought involved in the repair of the delicate spiral capillaries under the nail, which are subject to frequent injury.
Depigmented or white hair is usually noticed after a month of treatment with sunitinib. It is reversible with restoration of hair colour two to three weeks after treatment is stopped.
Kinase inhibitors may lead to local or diffuse hypopigmentation, drug-induced vitiligo (white skin, leukoderma), and hyperpigmentation (dark patches).
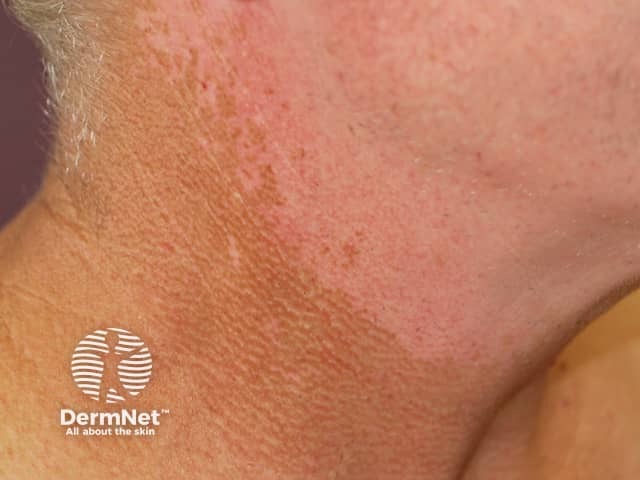
Leukoderma due to protein kinase inhibitor
Protein kinase inhibitors may lead to sunburn after minimal exposure to the sun (drug-induced photosensitivity).
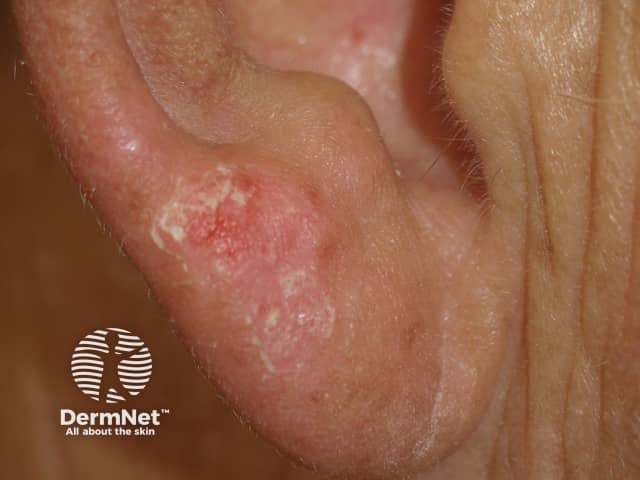
Sunburned earlobe following minimal sun exposure due to vemurafenib