Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: July, 2025
Authors: Nancy Huang (MBChB), DermNet Medical Writer, New Zealand (2025); Dr Estella Janz-Robinson, Dermatology Registrar, Australia (2021); Hana Numan, DermNet Medical Writer, NZ (2022 and 2023). Minor update November 2025.
Peer reviewed by: Dr Vidette Wong, Specialist doctor, United Kingdom (2025)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department.
Introduction
Uses
Contraindications and precautions
More information
Benefits
Disadvantages
Side effects and risks
Janus kinase (JAK) inhibitors, also called jakinibs or JAKis, are small molecules that interrupt the JAK-STAT (Signal Transducer and Activator of Transcription) signalling pathways involved in the pathogenesis of many immune-mediated inflammatory diseases (IMIDs).
Janus kinase inhibitors have shown beneficial effects in a variety of immune-mediated conditions.
JAK inhibitors have also been used successfully in conditions with JAK mutations such as polycythaemia vera, essential thrombocythaemia, and myelofibrosis.
Janus kinase inhibitors have been approved for use in or are currently under investigation for the following indications:
Indication |
Approved for use |
Under investigation |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Chronic hand dermatitis |
|
|
|
||
|
||
|
||
|
||
Granulomatous disorders including sarcoidosis, granuloma annulare, necrobiosis lipoidica |
|
Tofacitinib received FDA approval for the treatment of rheumatoid arthritis in 2012, and in 2017/18 also for psoriatic arthritis and ulcerative colitis. It is used off-label for psoriasis, and is currently under investigation for the treatment of several dermatological conditions including atopic dermatitis and vitiligo.
Ruxolitinib was approved by the TGA in Australia in 2013 for the treatment of myelofibrosis. It also became FDA-approved for the topical treatment of mild to moderate atopic dermatitis (2021), and non-segmental vitiligo in adult and paediatric patients 12 years of age and older (2022).
Baricitinib was TGA-approved in Australia in 2018 for the treatment of rheumatoid arthritis and, in 2021, for moderate to severe atopic dermatitis. Approval for this indication has also been granted by the European Union and Japan.
Upadacitinib was approved by Medsafe New Zealand (2021) and by the FDA (2022) for the treatment of moderate to severe atopic dermatitis. Oral abrocitinib and upadacitinib received licensing for use in atopic eczema in the USA and Europe in 2023.
In 2020, delgocitinib cream was approved in Japan for the treatment of atopic dermatitis in adults. In 2024, it received European approval for the treatment of moderate-to-severe chronic hand dermatitis, followed by FDA approval in 2025.
The Janus kinase family consists of four intracellular tyrosine kinases — JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). The JAKs are associated with transmembrane cytokine receptors and are activated by the binding of cytokines and growth factors.
When activated, JAKs phosphorylate the transmembrane cytokine receptor, leading to the activation of intracytoplasmic STATs. STATs then translocate to the nucleus and modulate gene transcription. The phosphate is provided by ATP, and each JAK and tyrosine kinase recognises a slightly different ATP-binding pocket. Overexpression of the JAK/STAT pathways can result in the development of autoimmune disease and malignancies.
JAK inhibitors (JAKis) can be classified according to their selectivity for specific JAK isoforms:
The availability of different JAK inhibitors varies across countries and regions.
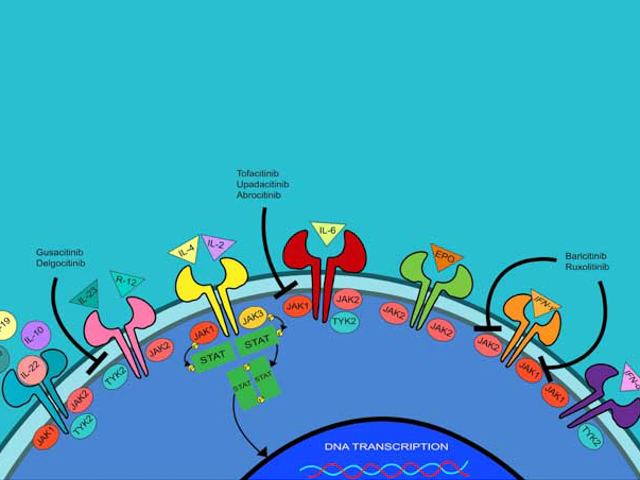
As Janus kinase inhibitors are small molecules, they can be used topically or orally, unlike biologic agents which require administration by injection. They are also significantly cheaper than biologics, which require complex biomanufacturing, as JAK inhibitors are chemically synthesised.
Based on head-to-head comparisons and meta-analyses, JAK inhibitors appear to demonstrate a faster onset of action than biologics in the treatment of atopic dermatitis.
Janus kinase inhibitors may interrupt the signal transduction of multiple cytokine receptors rather than target specific cytokines or cytokine receptors, leading to greater adverse effects.
For a number of dermatological conditions, clinical trials have shown rapid relapse is common after discontinuation of the JAK inhibitor such as alopecia areata, vitiligo, and psoriasis.
Common adverse side effects of JAK inhibitors include:
Janus kinase inhibitors increase susceptibility to serious bacterial, fungal, mycobacterial, and viral infections due to immune modulation. Screening for tuberculosis, hepatitis B and C, and HIV should therefore be undertaken before starting treatment with a JAK inhibitor.
Herpes virus reactivation appears to be common; patients should receive zoster vaccination (recombinant zoster vaccine) before initiating JAKi therapy. Antiviral prophylaxis may be considered for patients with recurrent herpes flares.
Vaccination recommendations:
See: Immunisation in immunosuppressed dermatology patients.
If signs or symptoms of serious infection develop during treatment with JAKis, treatment should be stopped until the infection is controlled.
JAKis are associated with increases in lipid parameters (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) during the first 12 weeks of treatment; lipid levels tend to stabilise thereafter. Dyslipidaemia should be managed according to clinical guidelines.
There are inconsistently reported associations between JAKis and an increased risk of MACEs (composite of non-fatal myocardial infarction, non-fatal ischemic stroke, and cardiovascular death).
Tofacitinib was associated with a higher risk of MACEs compared to tumour necrosis factor (TNF) inhibitors in the treatment of patients aged ≥50 years with rheumatoid arthritis. However, this increased risk was primarily observed in patients aged ≥65 years and those with pre-existing risk factors eg, atherosclerotic disease, long-time smoking.
Although similar adverse events have not yet been reported with baricitinib and upadacitinib, the FDA found the risks have not been adequately evaluated and issued a black box warning due to the similar mechanisms of action.
An association between JAKis and malignancy (eg, T-cell lymphoma) is currently under investigation, with results to date being mixed.
For example, a 2023 meta-analysis found an increase in malignancy risk for JAKis compared to tumour necrosis factor inhibitors, but not when compared to placebo or methotrexate.
Tofacitinib was found to be associated with an increased incidence of malignancy compared to TNF inhibitors for rheumatoid arthritis patients aged ≥50 years with at least one cardiovascular risk factor. The TGA in Australia warns high dose (10mg bd) should be used with caution in patients with non-melanoma skin cancer.
Certain JAKis may be associated with an increased risk of arterial and venous thromboembolism (VTE), particularly tofacitinib and baricitinib.
JAKis have been associated with lymphopenia, neutropenia, and anaemia. These effects may be more common in non-selective JAKis.
Treatment should be interrupted if patients develop an absolute lymphocyte count <0.5 × 109/L, absolute neutrophil count <1 × 109/L, or haemoglobin concentration <80 g/L. Treatment may be restarted once levels return above these thresholds.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).