Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: June, 2025
Author(s): Uladzislau Barabash, Lancaster University, United Kingdom (2025)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department.
Introduction
Uses
How it works
Precautions and contraindications
Administration and monitoring
Benefits
Disadvantages
Side effects and risks
Deucravacitinib is an oral, selective tyrosine kinase 2 (TYK2) inhibitor for the systemic treatment of moderate to severe plaque psoriasis and other immune-mediated diseases.
Deucravacitinib (SOTYKTU™) was first approved in September 2022 by the US Food and Drug Administration (FDA) and subsequently in the European Union (EU) for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Deucravacitinib was later approved in Japan for treatment of plaque psoriasis, generalised pustular psoriasis, and erythrodermic psoriasis.
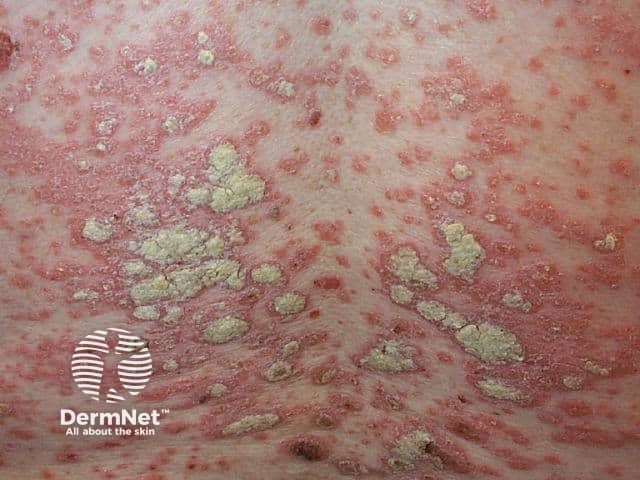
Chronic plaque psoriasis
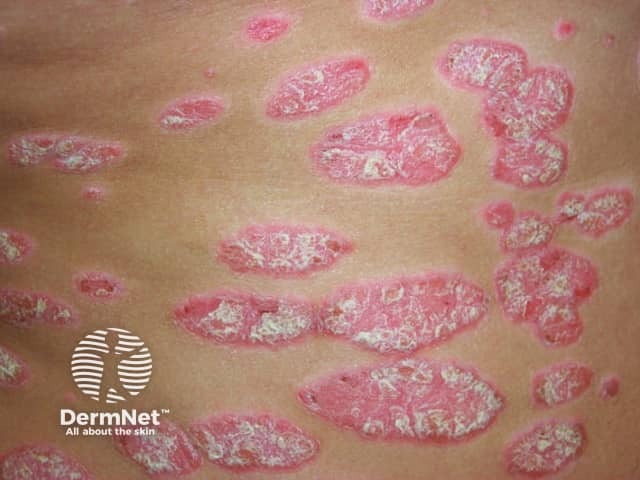
Chronic plaque psoriasis
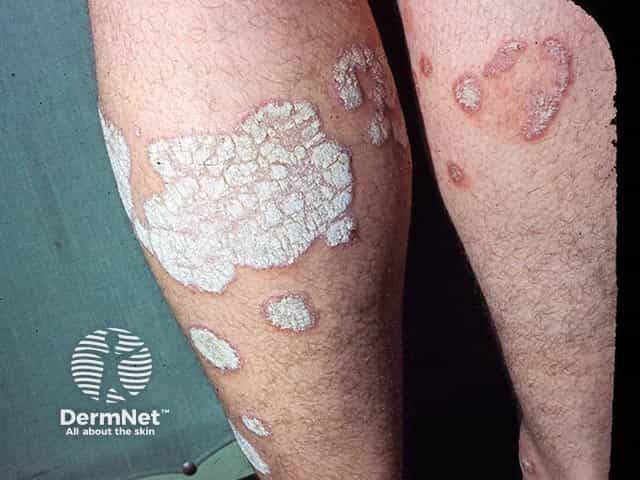
Thick scale overlying plaques of psoriasis on the leg
Further clinical trials are underway to explore the efficacy of deucravacitinib for treatment of other immune-mediated diseases such as:
Deucravacitinib is the first in a new class of oral, selective tyrosine kinase 2 (TYK2) inhibitors.
TYK2 is an intracellular enzyme and part of the Janus kinase (JAK) family. The JAK family is essential for cytokine signalling.
While JAK inhibitors competitively bind to the catalytic domain, which is highly conserved across the JAK family, deucravacitinib allosterically binds the regulatory domain unique to TYK2, thus driving its selectivity for TYK2 without inhibiting JAK1/2/3 at clinically relevant doses.
The precise mechanism of action explaining the link between TYK2 inhibition and therapeutic effectiveness of deucravacitinib is currently unknown.
It is postulated that TYK2 pairs with other Janus kinase enzymes to mediate signalling of various pro-inflammatory cytokines eg, interleukin-23 (IL-23), IL-12, and Type I interferons (IFNα/β).
Deucravacitinib is a systemic therapy taken as a once-daily oral tablet.
Monitoring:
Clinical trials extending up to 52 weeks have shown that deucravacitinib is generally well tolerated.
Common side effects:
Mucocutaneous side effects:
Uncommon side effects:
Serious adverse events:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and [British National Formulary for Children ](http://bnfc.nice.org.uk/ (BNFC).