Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author(s): Dr Libby Whittaker, Medical Writer, DermNet; William Ju, University of Otago, New Zealand (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Precautions and contraindications
Use in specific populations
Benefits
Disadvantages
Side effects and risks
Ruxolitinib is a selective Janus-activated tyrosine kinases inhibitor (JAK1 and JAK2), available in oral (Jakafi™) and topical (Opzelura™) formulations.
It is used to treat myelofibrosis, graft-versus-host disease, and in dermatology, atopic dermatitis, and non-segmental vitiligo.
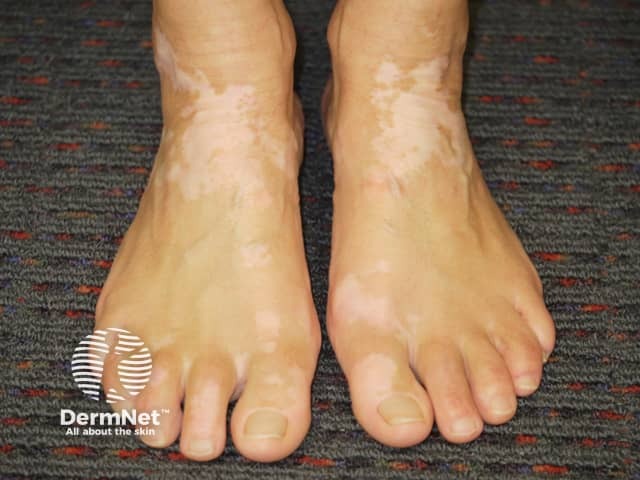
Ankle vitiligo suitable for ruxolitinib cream
Oral ruxolitinib is approved for the treatment of:
Topical ruxolitinib is approved for the treatment of the following conditions in adults and children ≥12 years old:
Other potential uses currently under investigation include:
Janus kinase (JAK) 1 and 2 mediate the signalling of cytokines and growth factors which are important for the process of haematopoiesis and immune function. Janus kinase inhibitors downregulate these inflammatory cytokines, which can be a useful mechanism of action in a variety of immune-mediated conditions including graft-versus-host disease, atopic dermatitis, and vitiligo.
Other precautions for oral ruxolitinib:
Other precautions for topical ruxolitinib:
Recommended starting dose:
After six months of treatment, tapering can be trialled every 8 weeks from 10 mg oral twice daily, to 5 mg twice daily, to 5mg once daily.
Topical ruxolitinib is available as 1.5% cream. Recommended dosing:
Population |
Recommendation |
|
Pregnancy |
|
|
Breastfeeding |
|
|
Paediatric |
|
|
Geriatric |
|
|
Renal impairment |
|
|
Hepatic impairment |
|
|
Clinical trial |
Response |
Phase 2 trial for acute GVHD |
39/71 patients reached overall response after 28 days and 19 patients achieved complete response. |
Phase 3 trial for acute GVHD |
Overall response rate at 28 days was higher in the ruxolitinib group (96/154) than the control group (61/155). |
Phase 3 trial for chronic GVHD |
Overall response rate at 24 weeks was higher in the ruxolitinib group (82/165) than the control group which received best available other therapy (42/164). |
Adverse effects are more common for oral than topical ruxolitinib, and may include:
Other serious potential adverse effects may include:
Higher rates of other malignancies (eg, lymphoma), thromboembolic events (eg, deep vein thrombosis, pulmonary embolism), and other adverse cardiovascular events (eg, myocardial infarction or stroke) were observed in a study comparing another Janus kinase inhibitor, tofacitinib, to tumour necrosis factor inhibitors for rheumatoid arthritis.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).