Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Last Reviewed: November, 2025
Author(s): Maitreyi Aria Jain, The University of Auckland; Honorary Associate Professor Paul Jarrett, Dermatologist, Middlemore Hospital and Department of Medicine, The University of Auckland, Auckland, New Zealand (2025)
Peer reviewed by: Dr Eloise James, Monash Health, Australia (2025)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
Contraindications
More information
Side effects and risks
Bruton kinase inhibitors (BTKIs) are drugs that interfere with the B-cell receptor signalling pathway, inhibiting the proliferation and survival of malignant B cells.
BTKIs are most commonly used in haematological conditions, treating certain leukaemias and lymphomas, including chronic lymphocytic leukaemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL) and, more recently, Waldenström macroglobulinaemia. There is growing research into the use of BTKIs for immune-mediated conditions, including those within dermatology.
In 2025, oral remibrutinib received FDA approval for the treatment of chronic spontaneous urticaria in adults unresponsive to H1 antihistamine therapy.
BTK inhibitors are absolutely contraindicated in breastfeeding and pregnancy.
BTKIs are considered relatively contraindicated, or to be used with caution, in patients with established cardiovascular disease, due to concern of precipitating atrial fibrillation, hypertension, and heart failure.
First generation BTKIs were developed and approved for use for haematological conditions in the USA around 2013. Ibrutinib is a first generation BTKI which binds irreversibly leading to inhibition of B cell receptor signalling and other “downstream” effects. Acalabrutinib and zanubrutinib are second generation BTKIs and are more selective inhibitors.
Research into the use of BTKIs in immune-mediated dermatological conditions is ongoing, including use for conditions such as:
Fenebrutinib is in active development in urticaria, branebrutinib in atopic dermatitis and systemic lupus, and rilzabutinib in pemphigus.
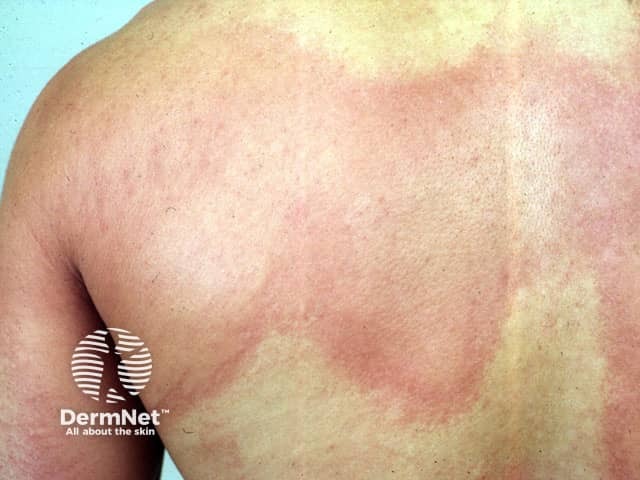
Chronic spontaneous urticaria - an evolving indication for BKIs
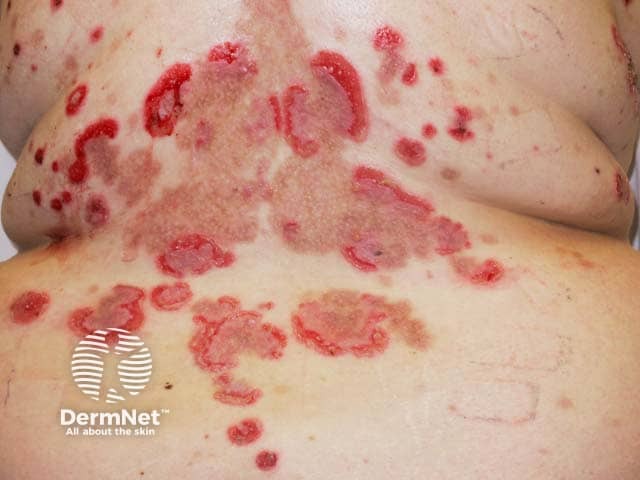
Pemphigus vulgaris - an evolving indication for BKIs (PV-patient3)
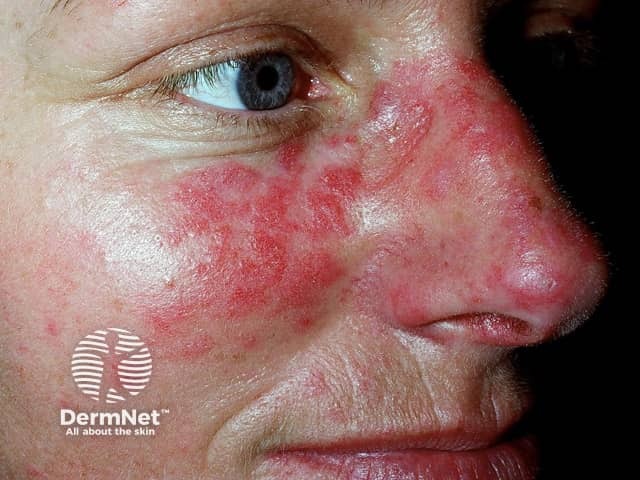
Systemic lupus erythematosus - an evolving indication for BKIs
There are a broad range of cutaneous side effects as a result of BTKIs, many of which are mild and may not lead to treatment discontinuation. Some reactions, however, can be severe and warrant treatment cessation.
Bleeding - minor, 30% patients have been reported to experience skin bleeding events
Mild Rash
Severe Rash
Peripheral oedema - “tree trunk legs”
Opportunistic infection/cellulitis
Some studies have suggested an increased risk of primary skin cancer
Nails and hair
Mucositis
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).