Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Reactions Blood vessel problems
Author: Dr Delwyn Dyall-Smith FACD, Dermatologist, 2010.
Introduction
Demographics
Associated medications
Clinical features
Diagnosis
Treatment
Drug-induced lupus erythematosus is an uncommon, mild to moderately severe, a lupus-like syndrome related in time to continuous exposure to a specific medication and which resolves after the trigger drug is ceased. As in idiopathic or usual-type lupus erythematosus (LE), there are three categories recognised:
This excludes medication-induced flares of pre-existing or latent LE.
Drug-induced LE differs from idiopathic LE.
There is a time relationship with the causative drug: the symptoms must have begun after starting treatment with a drug. But the latent period (lag or incubation time) between starting the medication and the first symptoms ranges from 1 month to more than ten years. The delay in onset can make it difficult to identify the trigger drug.
It has been estimated that 10% of cases of SLE are drug-induced and, in one case series, 20% of biopsy-proven cases of subacute cutaneous LE were drug-induced.
There are some predisposing genetic factors identified, including:
The drugs associated with drug-induced LE can be classified into four groups:
Another way to classify them is as high, medium, low or very low risk.
The most commonly reported medications reported to have a definite association with drug-induced SLE are:
The most frequently reported trigger drugs for drug-induced subacute cutaneous LE include:
Medications reported to cause drug-induced chronic cutaneous LE include:
Drug-induced SLE begins with a gradual onset of generally mild lupus-like symptoms:
Lupus-specific skin changes are rare in drug-induced SLE, but the following may infrequently occur in mild forms:
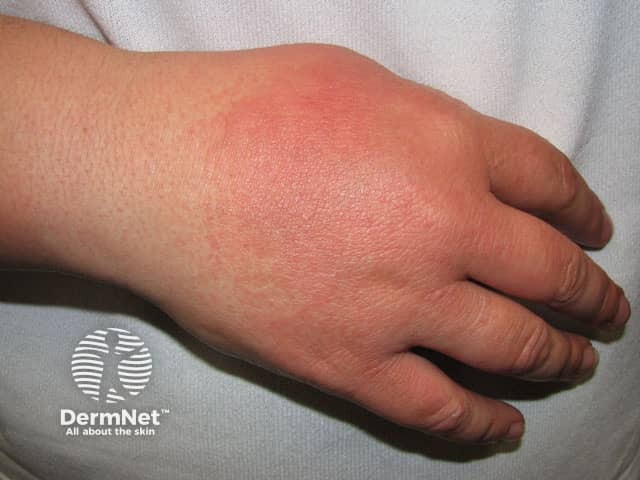
Photosensitivity
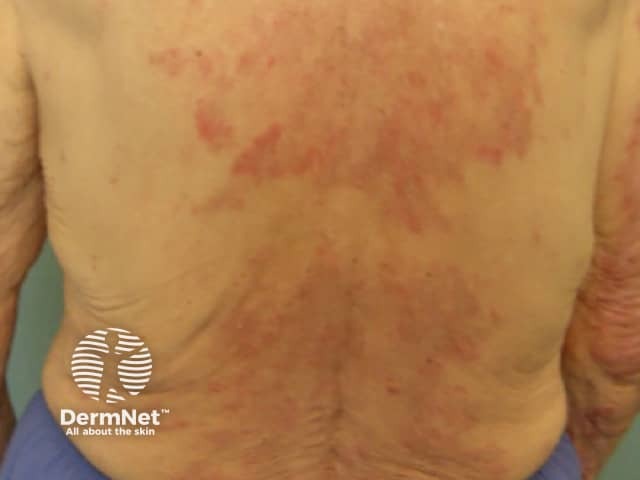
Urticarial vasculitis
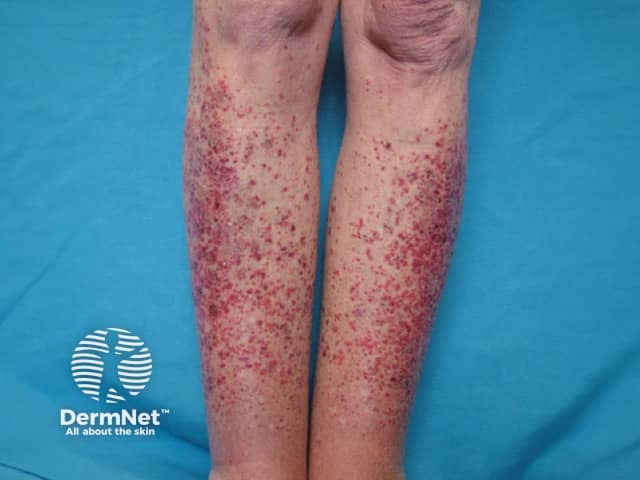
Hypersensitivity vasculitis
Common features of idiopathic SLE that are rare or absent from drug-induced SLE include:
Drug-induced SCLE cannot be distinguished from the idiopathic form as there are no significant differences in clinical, histological, immunological or laboratory features. However, it should be suspected in an older person developing this for the first time. The incubation period has been reported to range from 2 weeks to over three years.
The skin rash is typical of SCLE with symmetric nonscarring annular or polycyclic (ring-shaped) or papulosquamous (raised scaly) lesions usually on sun-exposed areas. However, in drug-induced SCLE the rash can be more widespread than in the idiopathic form, including involvement of the lower legs. The rash may be blistering, particularly at the edges of active lesions. Clinically relevant internal organ involvement as seen in SLE has been absent or minimal in reported cases.
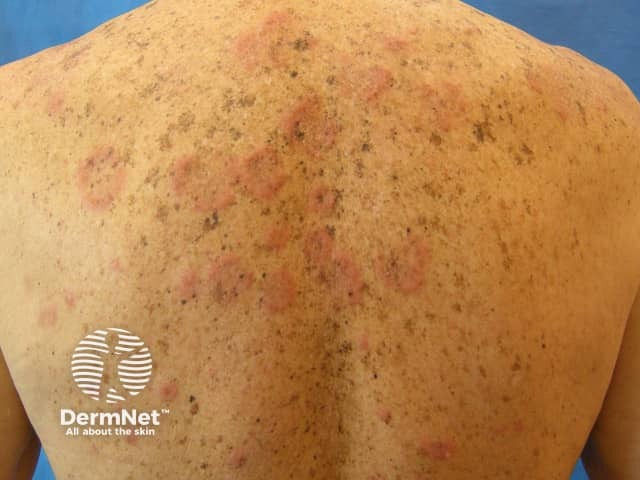
Drug-induced subacute lupus erythematosus
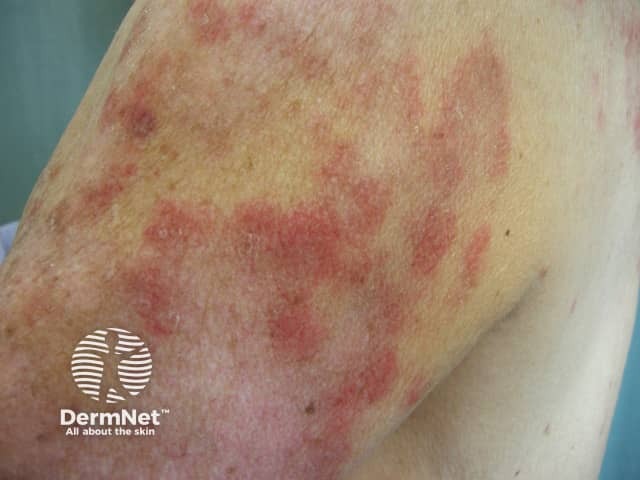
Drug-induced subacute lupus erythematosus
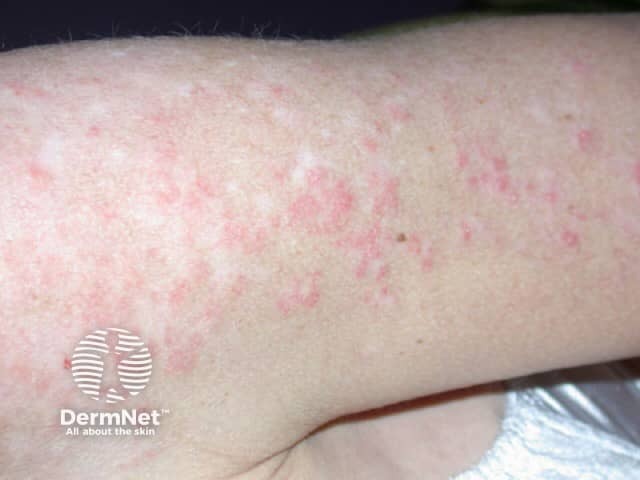
Drug-induced subacute lupus erythematosus
Drug-induced chronic cutaneous LE is the rarest of the three forms. It appears on average, eight months after starting the trigger medication. Males and females have been equally affected, and the mean age of onset has been 59 years.
Lesions resembling discoid lupus erythematosus (DLE) have been the most common presentation, but LE tumidus has been reported. The lesions typically affect the face, upper trunk and arms.
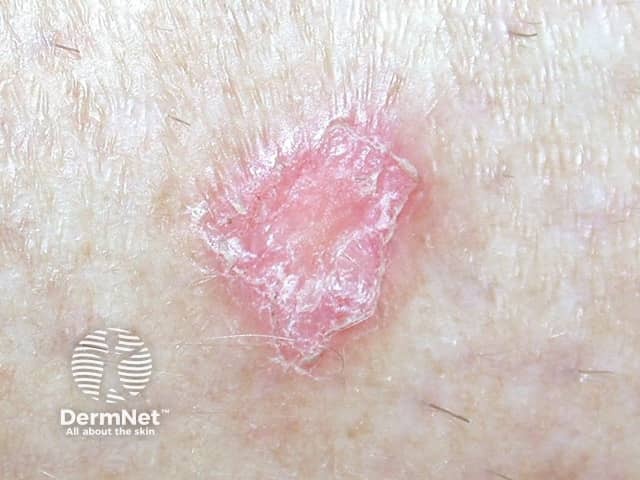
Discoid LE
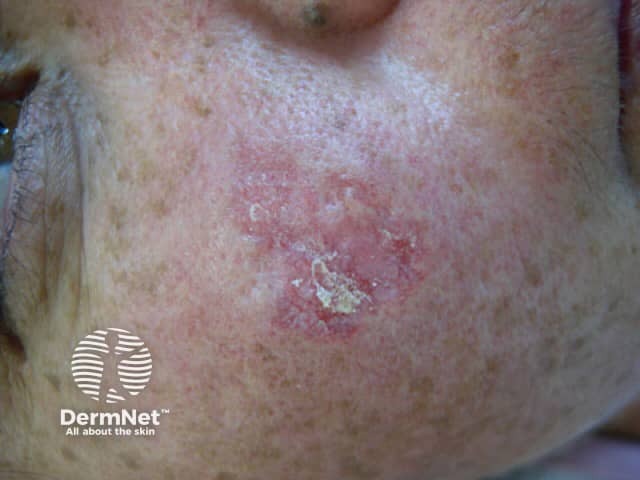
Discoid LE
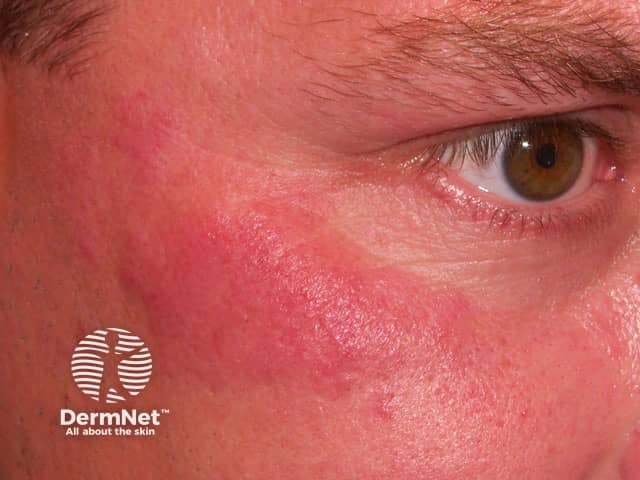
Lupus tumidus
It is important the diagnosis be considered. For example, the first onset of typical subacute cutaneous LE in an older person, especially if involving the legs, should raise the suspicion of drug-induced LE.
There are no standard diagnostic criteria for drug-induced LE at this time. Most patients would not fulfil the American Rheumatologic Association (ARA) criteria for diagnosing SLE.
Proposed criteria are:
Investigations would usually be done, including blood tests and skin biopsy.
Drug-induced SLE
Drug-induced subacute cutaneous LE
Drug-induced chronic cutaneous LE
The dermatopathology of a skin biopsy: histology and direct immunofluorescence are indistinguishable from idiopathic forms.
The most important treatment is to stop the trigger drug as this leads to resolution of the symptoms and blood test abnormalities. However, this can be difficult to identify if the patient is on many medications as the incubation period is so variable and can be very long. It may require carefully supervised ‘drug holidays’ of at least three months for each drug.
Unfortunately, at this time, there is no test to identify the drug apart from noting improvement when the drug is ceased and recurrence of symptoms within 1–2 days when rechallenged. Rechallenge, however, may not be recommended especially if internal organs have been affected.
Generally, the symptoms improve within weeks of stopping the drug, although full recovery may take as long as one year. The blood tests usually return to normal more slowly.
Sun protection should be advised where there is a sun-sensitive pattern to the rash.
Symptomatic relief may be required. This may include non-steroidal anti-inflammatory drugs (NSAID) for arthritis, topical steroids for rashes and systemic medications including hydroxychloroquine or oral corticosteroids such as prednis(ol)one for internal organ involvement. In most cases, no specific treatment is required as the drug-induced LE has been mild and resolves with drug withdrawal.
In general drug-induced, LE is milder than idiopathic LE, but life-threatening complications can occur (e.g. cardiac tamponade due to pericarditis) and it may be fatal if not recognised.