Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Lydia Chan, Medical Registrar, Waikato Hospital, Hamilton, New Zealand. Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, January 2015.
Introduction
Uses
How it works
Sirolimus ointment
Dosage
Monitoring
Drug interactions
Side effects
Prevention of infection
Sirolimus (trade name Rapamune™) is a selective immune-suppressing drug. It is most commonly used in patients that have a transplanted kidney to prevent their body from rejecting the new organ.
The drug is derived from a bacterium called Streptomyces hygroscopicus, which was found in the 1970s on the island Rapa Nui, hence its alternate name, rapamycin.
The main use of sirolimus is in patients with a kidney transplant. It may be chosen because other immune-suppressing drugs are causing side effects, or the patient is developing cancers associated with lowered immunity (including skin cancers). It is often used in combination with other immune-suppressing drugs such as ciclosporin or tacrolimus.
Oral sirolimus is also used off label in the treatment of graft versus host disease. It is under investigation for treating certain cancers and other diseases.
There have been small studies showing sirolimus may be effective in the following skin conditions:
The effect of sirolimus was often discovered when patients with these conditions required a kidney transplant and took sirolimus as part of their immune-suppressing drug regimen.
Sirolimus dampens down the body’s immune response by its action on lymphocytes (white blood cells). This reduces the body’s inflammatory response.
The mammalian target of rapamycin (mTOR) is a complex of protein kinases that promote cellular survival, cellular growth and catabolic processes. The mTOR pathway is inhibited by sirolimus/rapamycin. The mTOR pathway is also dysregulated in diabetes, obesity, some inflammatory skin diseases and certain cancers.
Sirolimus ointment has been trialled off-label to control facial angiofibromas in tuberous sclerosis.
The ointment has to be specially made up, usually by crushing a tablet of sirolimus, and mixing with a base such as petroleum jelly. Trials have varied the strength of the ointment (0.1 to 1%) and length of treatment/frequency of application; there is no current standardised treatment schedule. Patients using sirolimus ointment may also be treated with laser.
Oral sirolimus is available as a tablet and liquid. Starting dose is usually 6 mg, followed by 2 mg daily. Subsequently, the dose is varied according to the blood levels of the drug.
Occasionally, oral sirolimus is used at the same time as topical sirolimus.
Patients on oral sirolimus for renal transplant control should have the following checks.
Many medicines may interact with sirolimus; refer to the manufacturer’s data sheet for further information. Sirolimus is metabolised by the liver enzymes CYP3A4 and p-glycoprotein.
Skin |
|
|
General |
|
|
Mouth and gut |
|
|
Heart |
|
|
Infections |
|
|
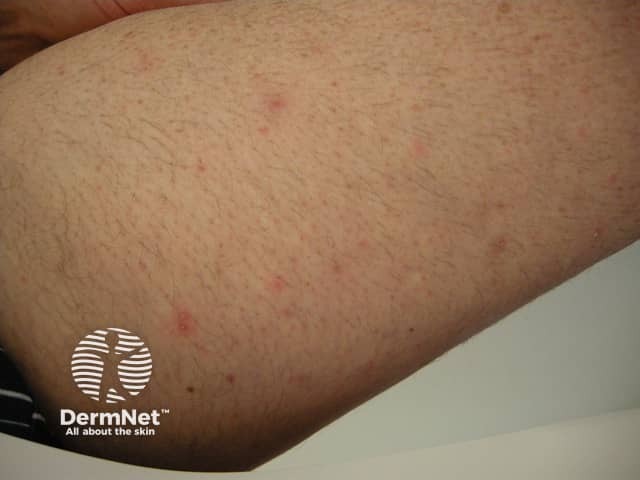
Folliculitis induced by sirolimus
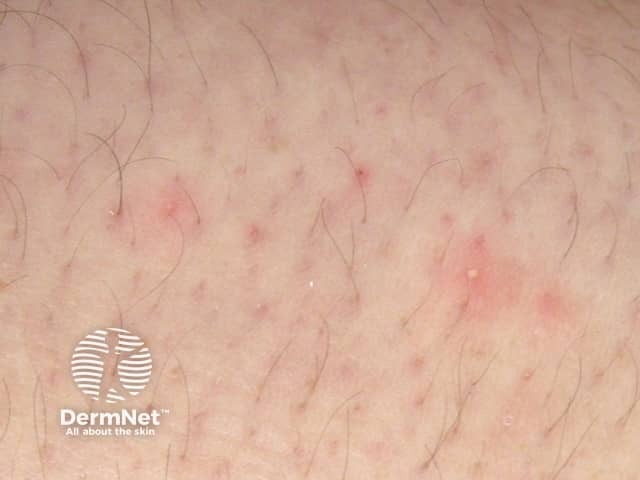
Folliculitis induced by sirolimus
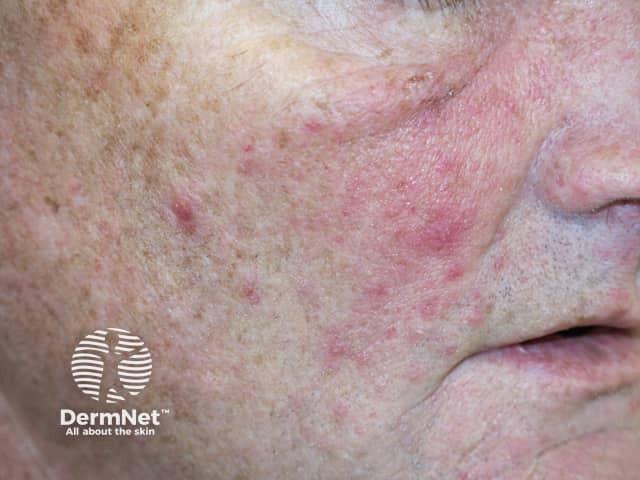
Folliculitis induced by sirolimus
Patients are generally screened for infections before starting sirolimus. These should include:
Immunisation may be less effective in patients on sirolimus due to a reduced immune response.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).