Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Introduction Inflammatory skin reactions Allergic reactions Viral infection
Most adverse cutaneous events associated with vaccines are caused by a normal inflammatory response to a foreign substance. As these inflammatory reactions are not related to allergy, most patients can receive subsequent vaccinations safely. A less common cause of an adverse skin reaction is an allergy to a vaccine or one of its components.
Vaccination/constituent |
Reaction |
Cause |
|---|---|---|
Most vaccinations |
Local injection site reactions such as swelling, redness and/or pain may occur with up to 80% of vaccine doses, depending on the type of vaccine. Local reactions generally occur within a few hours of the injection, are usually mild and do not require any specific treatment. |
The normal inflammatory response to a foreign substance. |
Measles, mumps, and rubella (MMR) vaccine |
Transient rash in up to 5% of recipients. |
As the vaccine contains live viruses, it is probably due to vaccine-induced modified measles. |
Varicella vaccine |
Varicella lesions may appear at the injection site in approximately 3% of recipients. In another 3%, the lesions may be more generalised. A zoster-type rash may rarely appear after varicella vaccination. |
Another live virus vaccine that can cause vaccine-induced illness. |
Tetanus and diphtheria toxoids |
Hypersensitivity (sometimes called Arthus-type) reaction — rare, severe local reaction (swelling, redness, pain). Not allergic. |
Believed to be due to the very high concentration of antibody, usually caused by too many doses of toxoid. |
Aluminium hydroxide |
Induces subcutaneous nodules (small lumps under the skin) in up to 19% of patients. Nodules usually resolve in a few months. Not allergic. |
Non-specific reaction to a foreign substance. |
Tetanus and diphtheria toxoids |
Generalised urticaria (hives), angioedema, and unidentified rashes in around 5–13% of recipients. |
Studies suggest that most mild to moderate generalised reactions result from non-specific activation of the inflammatory system by high doses of bacterial components. Subsequent booster injections of the suspected vaccines are well-tolerated. |
Localised allergic contact dermatitis can be induced by vaccine components. Historically, dermatitis was due to neomycin and thiomersal. Thiomersal is no longer present in vaccines in New Zealand but may be used in some vaccines elsewhere.
Contact allergy is confirmed by patch tests using potential allergens in the vaccine.
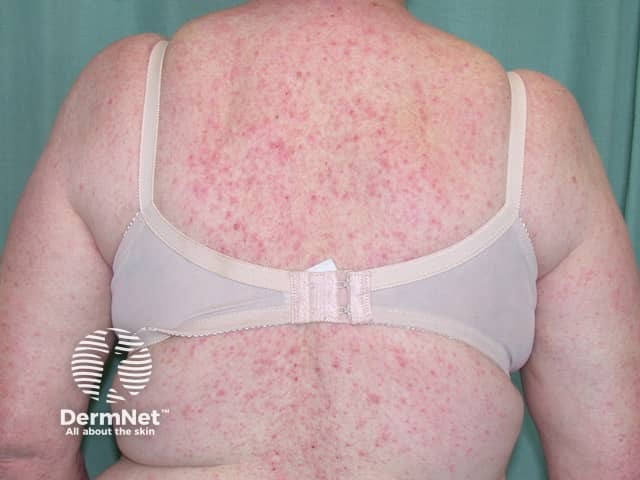
Dermatitis following influenza vaccine
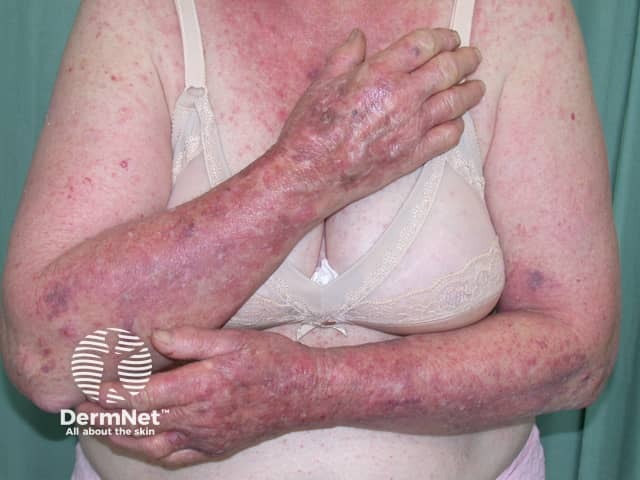
Dermatitis following influenza vaccine
The patient above had longstanding hand dermatitis and developed severe generalised dermatitis shortly after her first influenza vaccination.
Anaphylaxis is a severe allergic reaction that occurs at a rate of approximately 1 per million vaccine doses. The allergic reaction may be caused by the vaccine antigen itself or some other component of the vaccine such as animal protein, antibiotic, preservative, or stabiliser; gelatin, egg, chicken and/or yeast are commonly implicated.
Anaphylactic reactions usually occur within 4 hours of receiving a vaccination and involve multiple body systems, with generalised urticaria (hives), angioedema, difficulty breathing, and low blood pressure. Severe anaphylaxis can be life-threatening. If an anaphylactic reaction occurs, immediate resuscitation is required (see ASCIA anaphylaxis action plan). Later, allergy prick testing is recommended (under careful medical supervision) and further doses of the vaccine should not be given. A blood test called RAST or EAST testing can be used to exclude egg allergy.
Drug-induced urticaria without anaphylaxis can also result from vaccines.
Erythema multiforme is occasionally caused by vaccines. Erythema multiforme is characterised by target or bulls-eye lesions on hands, feet and lower legs. It has been reported after mumps/measles/rubella, diphtheria/pertussis/tetanus, hepatitis A/B, human papillomavirus (genital wart vaccine) and meningitis vaccinations.
Stevens-Johnson syndrome / toxic epidermal necrolysis has also rarely been reported to follow vaccination. This is a potentially very serious condition in which skin and mucous membranes blister and erode.
Vaccines are intended to prevent viral disease. However, patients that are on immunosuppressive therapy should avoid live vaccines in case the vaccine causes widespread viral disease. For example, herpes zoster vaccination is contraindicated in these patients due to the risk of disseminated herpes zoster infection, which can be fatal.