Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author(s): Michelle Rubbrecht, Medical Writer, Belgium (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Contraindications and precautions
Benefits
Disadvantages
Side effects and risks
Bimekizumab (Bimzelx®) is a novel humanised immunoglobulin (IgG1) monoclonal antibody used to treat moderate to severe plaque psoriasis, generalised pustular psoriasis, and psoriatic erythroderma.
Bimekizumab selectively and directly inhibits interleukin 17A and 17F (IL-17A and IL-17F).
It is produced in a genetically engineered Chinese hamster ovary (CHO) cell line by recombinant DNA technology.
Bimekizumab (Bimzelx®) was approved in the European Union and the United Kingdom in August 2021 for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
It received further marketing authorisation in Japan in January 2022 for the treatment of plaque psoriasis, generalised pustular psoriasis, and psoriatic erythroderma in patients who are not responding adequately to existing treatments.
Bimekizumab was also granted marketing approval in Canada and Australia in early 2022 for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Currently, bimekizumab is being evaluated in psoriatic arthritis, ankylosing spondylitis, axial spondyloarthritis, palmoplantar pustular psoriasis, and hidradenitis suppurativa.
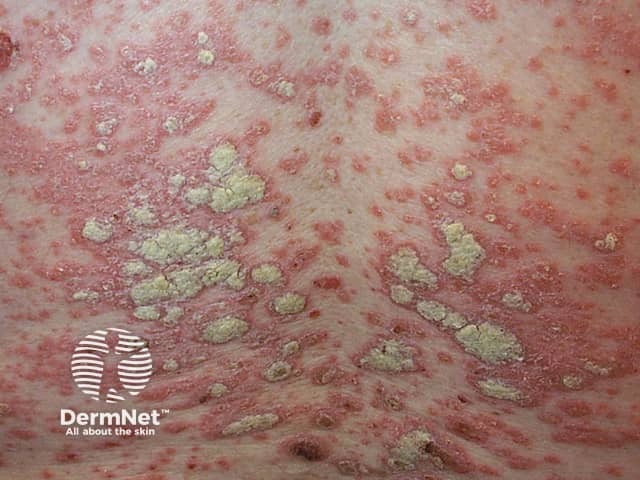
Chronic plaque psoriasis
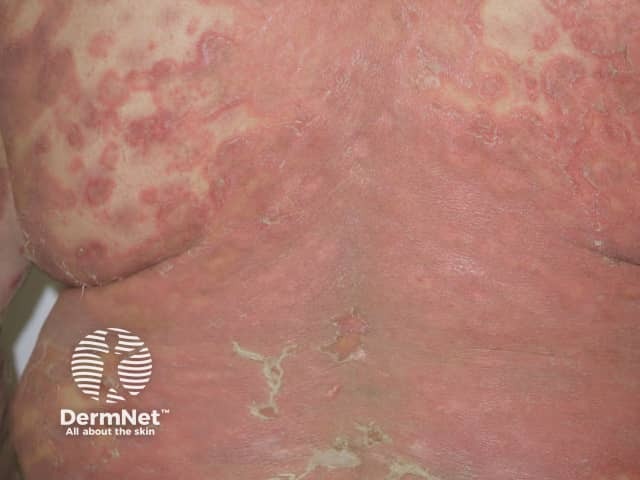
Generalised pustular psoriasis
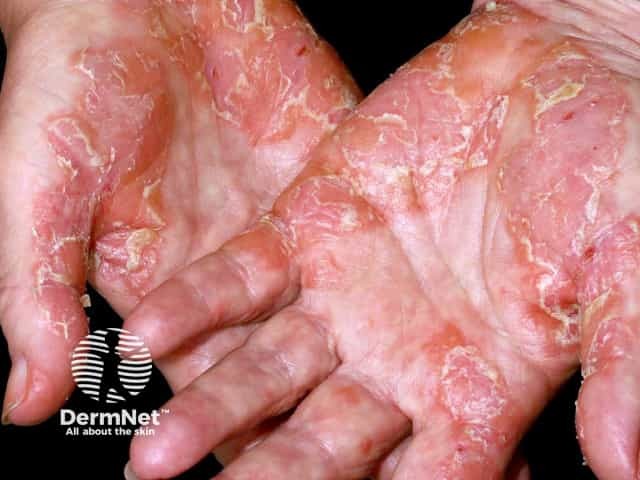
Palmar psoriasis
See also: Interleukin-17 in inflammatory skin disorders.
Bimekizumab is contraindicated in patients with:
Precautions |
|
|---|---|
Infections |
|
Pre-treatment screening |
|
Inflammatory bowel disease |
|
Hypersensitivity |
Discontinue treatment immediately in case of severe hypersensitivity or anaphylactic reaction; severe hypersensitivity reactions, including anaphylactic reactions, have been reported with IL-17 inhibitors. |
Vaccinations |
|
Pregnancy and lactation |
|
Contraception |
|
The most frequently reported side effect (>10%) of bimekizumab treatment is upper respiratory tract infections (eg, nasopharyngitis).
Common side effects (1-10%) include:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).